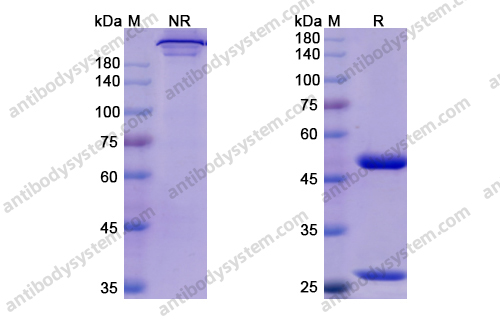
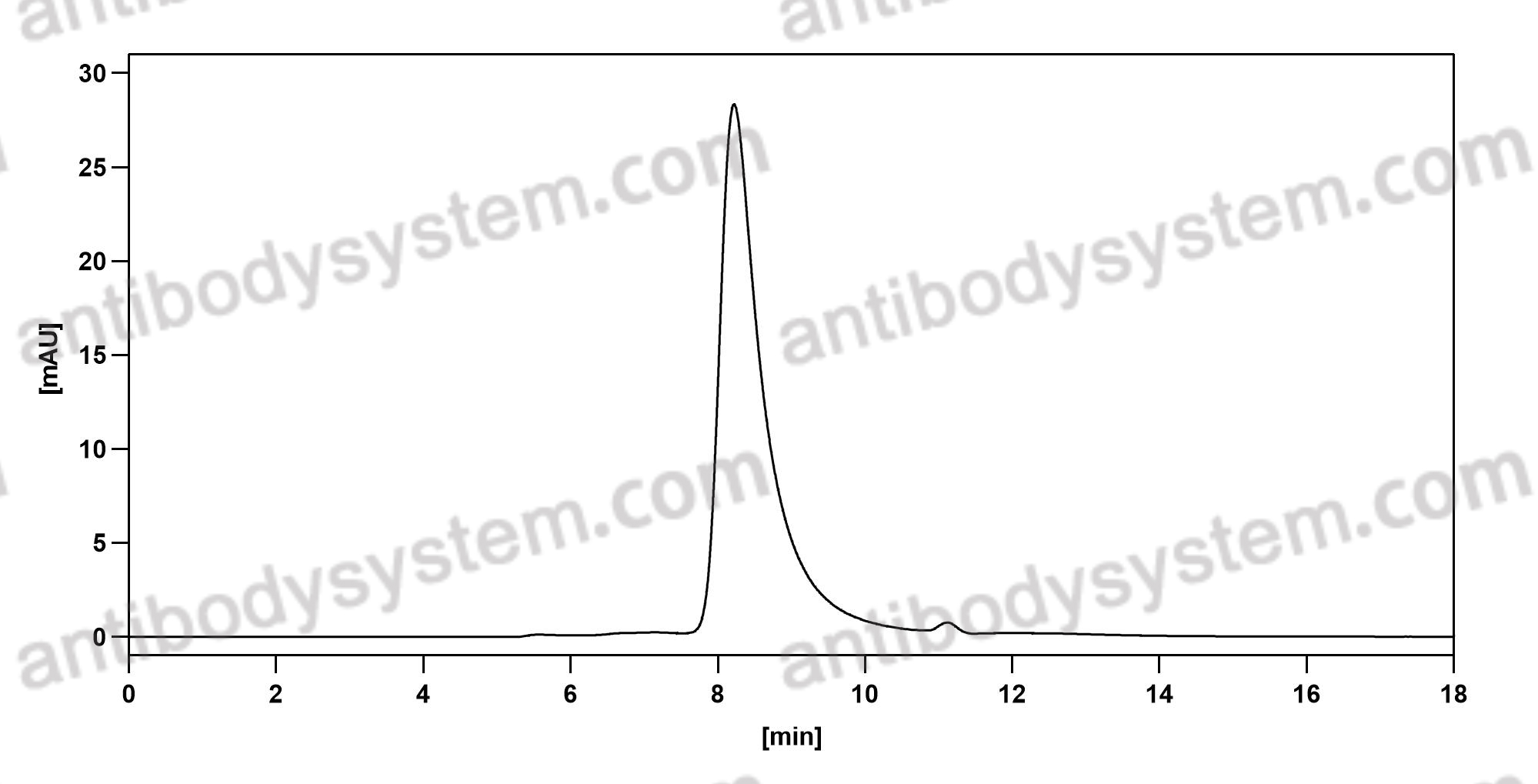
Risankizumab for the treatment of psoriasis, PMID: 31460804
Risankizumab: First Global Approval, PMID: 31098898
Risankizumab: A Review in Moderate to Severe Plaque Psoriasis, PMID: 32632826
Risankizumab: an anti-IL-23 antibody for the treatment of psoriasis, PMID: 30518998
Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis, PMID: 28423301
Risankizumab for psoriasis, PMID: 32346218
Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial, PMID: 32594522
Risankizumab in the treatment of psoriasis - literature review, PMID: 31462831
Update on risankizumab for the treatment of moderate to severe psoriasis, PMID: 32933320
Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials, PMID: 30097359
Risankizumab in patients with moderate to severe Crohn's disease: an open-label extension study, PMID: 30056030
Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial, PMID: 31280967
Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review, PMID: 32427307
Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials, PMID: 31583255
Psoriatic skin molecular and histopathologic profiles after treatment with risankizumab versus ustekinumab, PMID: 30578873
Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn's disease: a randomised, double-blind, placebo-controlled phase 2 study, PMID: 28411872
Efficacy and Safety of Continuous Risankizumab Therapy vs Treatment Withdrawal in Patients With Moderate to Severe Plaque Psoriasis: A Phase 3 Randomized Clinical Trial, PMID: 32267471
Clinical Pharmacokinetics and Pharmacodynamics of Risankizumab in Psoriasis Patients, PMID: 31758502
A drug safety evaluation of risankizumab for psoriasis, PMID: 32100591
Risankizumab, PMID: 31145575
Risankizumab in moderate-to-severe plaque psoriasis, PMID: 31578912
Effect of Risankizumab on Patient-Reported Outcomes in Moderate to Severe Psoriasis: The UltIMMa-1 and UltIMMa-2 Randomized Clinical Trials, PMID: 33052382
Efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the phase III UltIMMa-1 and UltIMMa-2 studies, PMID: 32320088
Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: Results from the SustaIMM phase 2/3 trial, PMID: 31237727
Risankizumab, an IL-23p19 Inhibitor for Psoriasis: A Review of the Current Literature, PMID: 32510689
Efficacy and safety of Risankizumab in moderate to severe psoriasis: A systematic review and meta-analysis, PMID: 33140468
Clinical Evaluation of Risankizumab-rzaa in the Treatment of Plaque Psoriasis, PMID: 32158251
Risankizumab for the treatment of moderate to severe psoriasis, PMID: 30462554
Risankizumab.rzaa: an interleukin monoclonal antibody antagonist binding to the p19 subunit of human IL-23 cytokine, PMID: 31720558
Efficacy and safety of risankizumab in psoriasis patients who failed anti-IL-17, anti-12/23 and/or anti IL-23: Preliminary data of a real-life 16-week retrospective study, PMID: 32761740
Antibodies to watch in 2020, PMID: 31847708
Risankizumab vs. ustekinumab for plaque psoriasis: a critical appraisal, PMID: 30632140
Spotlight on risankizumab and its potential in the treatment of plaque psoriasis: evidence to date, PMID: 30519540
Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis, PMID: 31672037
Efficacy of in-class interleukin-23 inhibitor switching: risankizumab following guselkumab failure in moderate-to-severe psoriasis treatment, PMID: 32998185
Risankizumab for psoriasis, PMID: 30097360
Risankizumab (Skyrizi) for psoriasis, PMID: 31170118
Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics, PMID: 30772098
Pharmacoeconomic Review Report: Risankizumab (Skyrizi): (AbbVie) [Internet], PMID: 31876996
Risankizumab: How to choose the right dose in clinical practice?, PMID: 33021745
Risankizumab, PMID: 34251778
Romosozumab-aqqg, upadacitinib, and risankizumab-rzaa, PMID: 31735342
Risankizumab-Aggravated Crusted Scabies in a Patient with Down Syndrome, PMID: 32378153
Risankizumab as a promising therapeutic approach in obese patients, PMID: 32196833
New Treatment Addressing the Pathogenesis of Psoriasis, PMID: 33050592
Acrodermatitis continua Hallopeau successfully treated by risankizumab, PMID: 33368238
Risankizumab vs. adalimumab for moderate-to-severe plaque psoriasis: a critical appraisal, PMID: 31899817
Clinical Review Report: Risankizumab (Skyrizi): (AbbVie) [Internet], PMID: 31393686
Comparison of Biologics and Oral Treatments for Plaque Psoriasis: A Meta-analysis, PMID: 32022825
Risankizumab: a game changer in Crohn's disease?, PMID: 30056029
Biologics targeting IL-23 in moderate-to-severe psoriasis: lessons learned from real-world use., PMID:40522209
Psoriasis vulgaris in patients with a recent history of neoplasia: safety of interleukin-23 inhibitors. A multicentre retrospective study., PMID:40521671
Development and validation of a novel Endoscopic ulcer Activity Score for Evaluation of Crohn's Disease (EASE-CD) using data from two randomised controlled trials., PMID:40505666
Treatment of refractory pityriasis rubra pilaris with biologic therapy: a case series., PMID:40488550
Understanding Psoriasis Patient Preferences for Biologic Dosing Frequencies: Insights From a Patient Survey., PMID:40476147
Interleukin-23 Inhibitors for Inflammatory Bowel Disease: Pivotal Trials and Practical Considerations., PMID:40465057
Guselkumab (Tremfya) - an IL-23 antagonist for Crohn's disease., PMID:40459404
The effect of IL-17 and IL-23 ınhibitors on hematological ınflammatory parameters in patients with psoriasis vulgaris., PMID:40455345
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Emerging Therapies for Palmoplantar Pustulosis with a Focus on IL-23 Inhibitors., PMID:40429269
Optimizing Biologic Therapy for the Prevention of Post-Operative Recurrence in Crohn's Disease: Current Evidence and Future Perspectives., PMID:40427059
Real-World Effectiveness, Safety, and Drug Survival of Risankizumab in Adult Patients with Plaque Psoriasis: A 3-Year Multicenter Retrospective Cohort Study., PMID:40413337
Ustekinumab in the Treatment of Crohn's Disease-A Narrative Review on Clinical Efficacy and Safety Profile., PMID:40407511
Evaluating changes in baseline characteristics and drug utilisation pattern in patients with moderate-to-severe psoriasis: findings from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) Cohort., PMID:40402160
Patient-reported real-world experience of risankizumab on-body device (OBD) for the treatment of Crohn's disease in the UK (COMMODUS)., PMID:40401545
Interleukin-23p19 inhibitors for the treatment of moderate-to-severe psoriasis: an expert opinion of real-world evidence studies in Europe., PMID:40396610
Clinical insights into IL-23 inhibition: risankizumab for Crohn's disease through a systematic review and meta-analysis of randomized controlled trials., PMID:40395763
With guselkumab, does the dual mechanisms to inhibit IL-23, help in ulcerative colitis?, PMID:40394835
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
A systematic review of tumor necrosis factor-α blockers, anti-interleukins, and small molecule inhibitors for dissecting cellulitis of the scalp treatment., PMID:40383754
Correction: Real-World Effectiveness of Risankizumab in Patients with Moderate-to-Severe Psoriasis: Interim Analysis from the VALUE Global Prospective Post-marketing Observational Study at 25 Months., PMID:40329056
Biologics for the Treatment of Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-analysis., PMID:40329054
Mirikizumab (Omvoh) - an IL-23 antagonist for Crohn's disease., PMID:40324965
Unveiling Risankizumab's Rare Side Effect: A Case of Severe Thrombocytopenia in Psoriatic Arthritis., PMID:40291297
A multicenter study of the real-world effectiveness and safety of risankizumab in Crohn's disease., PMID:40289770
Efficacy and Safety of IL-17 and IL-23 Inhibitors in Elderly Patients With Plaque Psoriasis: A Real-World Study., PMID:40285439
New Interleukin-23 Antagonists' Use in Crohn's Disease., PMID:40283885
New Therapeutic Challenges in Pediatric Gastroenterology: A Narrative Review., PMID:40281872
The role of interleukin inhibitors in the treatment of hidradenitis suppurativa; a systematic review of clinical trials., PMID:40268126
Active Switch From a Single Dose of Bimekizumab to Risankizumab in Psoriasis: A Pilot Study., PMID:40260930
Use and Cost of First-Line Biologic Medications to Treat Plaque Psoriasis in the US., PMID:40238112
Severe Epistaxis Due to Risankizumab: A Case Report., PMID:40230732
Risankizumab-Rzaa in the Management of Birdshot Chorioretinopathy: A Case Report., PMID:40208207
Effect of Risankizumab Induction and Maintenance Therapy on the Rate of Hospitalization in Patients with Crohn's Disease., PMID:40190340
Exploring the Impact of Guselkumab and Risankizumab on Psoriasis in HIV-Positive Patients: Insights From Four Italian Centers., PMID:40176606
Corticosteroid-sparing effects of risankizumab efficacy and safety in patients with moderately to severely active ulcerative colitis., PMID:40168091
Correction to: P0722 Efficacy and Safety of Risankizumab in Patients With Moderate to Severe Crohn's Disease: Results From the One-Year SEQUENCE Open-Label Long-Term Extension., PMID:40147003
Guselkumab binding to CD64+ IL-23-producing myeloid cells enhances potency for neutralizing IL-23 signaling., PMID:40145093
Risankizumab Versus Secukinumab: A Real-World Efficacy and Cost per Responder Comparison in Patients With Psoriasis in Italy., PMID:40117618
Acyclovir-induced sinus bradycardia: a case report and literature review., PMID:40112886
Persistence and Efficacy of Ustekinumab in Crohn's Disease After Anti-TNF Failure: An Observational Study., PMID:40106111
Paradigm Shift in Inflammatory Bowel Disease Management: Precision Medicine, Artificial Intelligence, and Emerging Therapies., PMID:40095460
Symptom Resolution and Meaningful Improvement in Quality of Life With Risankizumab in Patients With Ulcerative Colitis: Post Hoc Analysis of the Randomized INSPIRE and COMMAND Studies., PMID:40094309
IL23p19 therapies for moderately-to-severely active ulcerative colitis., PMID:40082083
IL-23p19 Antagonists vs Ustekinumab for Treatment of Crohn's Disease: A Meta-Analysis of Randomized Controlled Trials., PMID:40071763
Clinical Experience of Risankizumab in Patients With a History of Erythrodermic Psoriasis., PMID:40071317
Guselkumab (Tremfya) for ulcerative colitis., PMID:40053377
Characteristics and long-term outcomes of children with perianal Crohn's disease., PMID:40038553
Safety of Interleukin Inhibitors in Psoriatic Patients with Latent Tuberculosis Infection Without Chemoprophylaxis: A Systematic Review., PMID:40026108