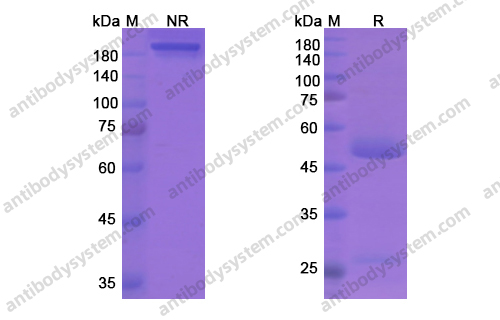
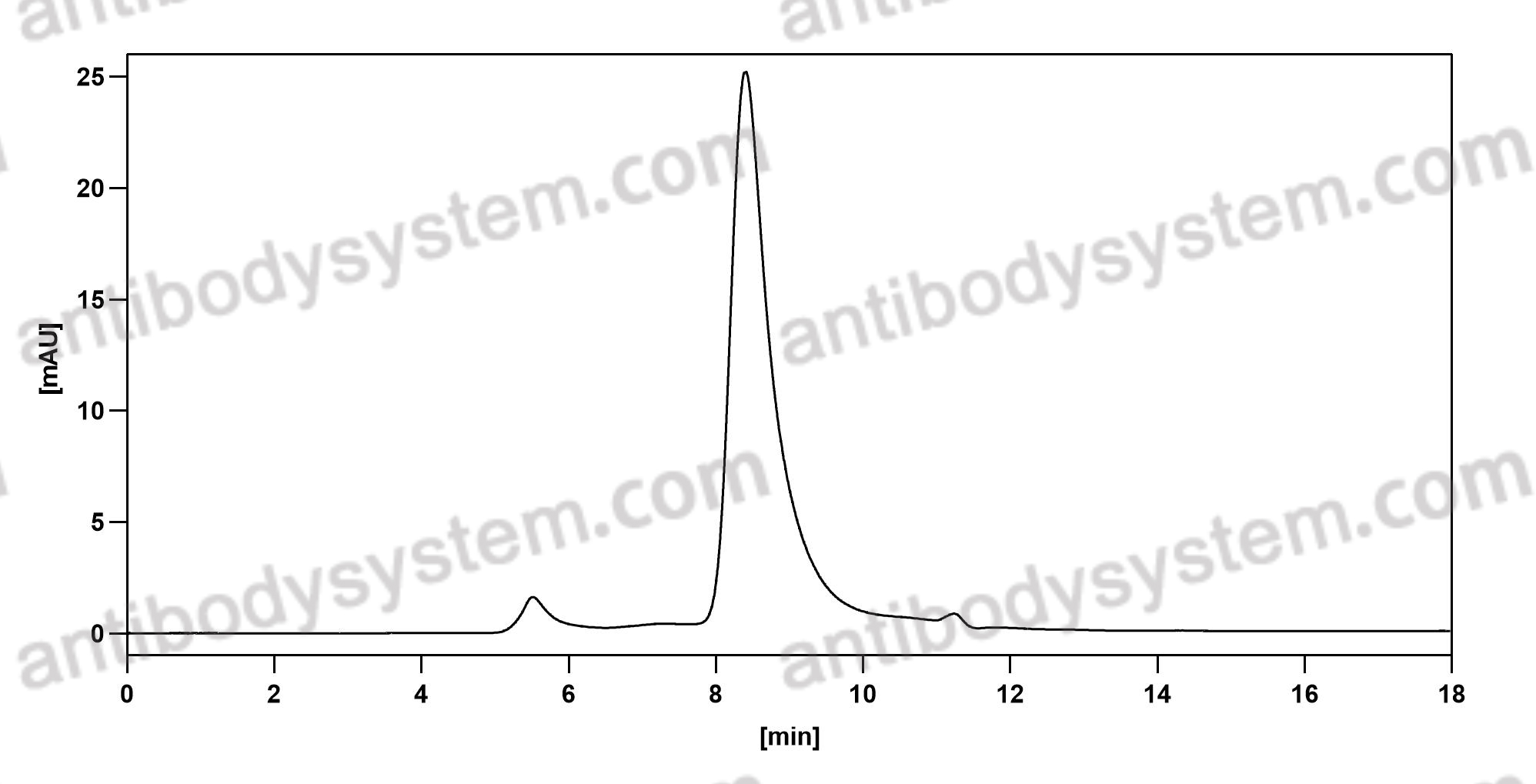
Fremanezumab for the Preventive Treatment of Chronic Migraine, PMID: 29171818
Fremanezumab: a disease-specific option for the preventive treatment of migraine, including difficult-to-treat migraine, PMID: 32832978
Fremanezumab for migraine, PMID: 32346217
Effect of Fremanezumab Compared With Placebo for Prevention of Episodic Migraine: A Randomized Clinical Trial, PMID: 29800211
Fremanezumab for the prevention of chronic and episodic migraine, PMID: 31050694
Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial, PMID: 31427046
Fremanezumab for the preventive treatment of migraine, PMID: 31177856
Long-term safety, tolerability, and efficacy of fremanezumab in migraine: A randomized study, PMID: 32913018
From LBR-101 to Fremanezumab for Migraine, PMID: 30311143
Fremanezumab as a preventive treatment for episodic and chronic migraine, PMID: 31043094
Fremanezumab, PMID: 31644089
An Evidence-Based Review of Fremanezumab for the Treatment of Migraine, PMID: 32222952
Fremanezumab for the preventive treatment of migraine in adults, PMID: 31220963
Early Onset of Efficacy With Fremanezumab for the Preventive Treatment of Chronic Migraine, PMID: 31675102
The impact of fremanezumab on medication overuse in patients with chronic migraine: subgroup analysis of the HALO CM study, PMID: 32958075
Fremanezumab (Ajovy) and galcanezumab (Emgality) for migraine prevention, PMID: 30681655
Fremanezumab, PMID: 30371997
No "Wearing-Off Effect" Seen in Quarterly or Monthly Dosing of Fremanezumab: Subanalysis of a Randomized Long-Term Study, PMID: 33009665
Migraine overview and summary of current and emerging treatment options, PMID: 30681821
Fremanezumab: First Global Approval, PMID: 30406901
Treatment benefit among migraine patients taking fremanezumab: results from a post hoc responder analysis of two placebo-controlled trials, PMID: 33413075
Safety profile of erenumab, galcanezumab and fremanezumab in pregnancy and lactation: Analysis of the WHO pharmacovigilance database, PMID: 33435709
Effect of fremanezumab on quality of life and productivity in patients with chronic migraine, PMID: 32747522
Fremanezumab for preventive treatment of migraine: Functional status on headache-free days, PMID: 30120138
A comprehensive overview and safety evaluation of fremanezumab as a preventive therapy for migraine, PMID: 32116061
Fremanezumab for migraine prevention: more effective, less costly, PMID: 32501385
Monthly versus quarterly fremanezumab for the prevention of migraine: a systemic review and meta-analysis from randomized controlled trials, PMID: 33136176
Fremanezumab in the treatment of migraines: evidence to date, PMID: 31686900
Safety and Efficacy of Fremanezumab for the Prevention of Migraine: A Meta-Analysis From Randomized Controlled Trials, PMID: 32508742
Effect of Fremanezumab Monthly and Quarterly Doses on Efficacy Responses, PMID: 32445498
Fremanezumab blocks CGRP induced dilatation in human cerebral, middle meningeal and abdominal arteries, PMID: 30109438
Do Treatment Strategies of Fremanezumab Have Similar Effect on Migraine?, PMID: 32011737
Fremanezumab inhibits vasodilatory effects of CGRP and capsaicin in rat cerebral artery - Potential role in conditions of severe vasoconstriction, PMID: 31589869
Monoclonal antibodies for the prevention of migraine, PMID: 31550937
Improvements across a range of patient-reported domains with fremanezumab treatment: results from a patient survey study, PMID: 32887548
Effects of fremanezumab on the use of acute headache medication and associated symptoms of migraine in patients with episodic migraine, PMID: 31752521
CGRP as the target of new migraine therapies - successful translation from bench to clinic, PMID: 29691490
CGRP Antibodies as Prophylaxis in Migraine, PMID: 30550780
Correction to: Fremanezumab: First Global Approval, PMID: 30859411
Safety and Tolerability of Fremanezumab for the Prevention of Migraine: A Pooled Analysis of Phases 2b and 3 Clinical Trials, PMID: 30977520
What's New in the Treatment of Migraine?, PMID: 31393282
Drugs for Migraine, PMID: 33434187
Population pharmacokinetic modelling and simulation of fremanezumab in healthy subjects and patients with migraine, PMID: 31418911
Current and emerging evidence-based treatment options in chronic migraine: a narrative review, PMID: 31470791
Role of CGRP in Migraine, PMID: 30725283
Fremanezumab and its isotype slow propagation rate and shorten cortical recovery period but do not prevent occurrence of cortical spreading depression in rats with compromised blood-brain barrier, PMID: 31895266
Fremanezumab as Add-On Treatment for Patients Treated With Other Migraine Preventive Medicines, PMID: 28862758
Cost of fremanezumab, erenumab, galcanezumab and onabotulinumtoxinA associated adverse events, for migraine prophylaxis in Spain, PMID: 32484365
Antibodies to watch in 2019, PMID: 30516432
Baloxavir marboxil, Fremanezumab-vfrm, Galcanezumab-gnlm, and Lofexidine hydrochloride, PMID: 30580925
Retrospective cohort study of anti-CGRP monoclonal antibody unresponsive migraine individuals treated with atogepant: The RESCUE study., PMID:40501126
A real-world study of the efficacy and tolerability of fremanezumab in migraine patients with a median follow-up of 14 months., PMID:40462186
Management of local and delayed cutaneous hypersensitivity reactions to anti-CGRP antibodies: implications for continuing treatment., PMID:40456979
Repurposed versus disease-specific medicinals for the prophylaxis of migraine: an updated systematic review., PMID:40447296
Postmarketing safety of migraine prophylactic monoclonal antibodies: An EudraVigilance database analysis of eptinezumab, fremanezumab, galcanezumab, and erenumab., PMID:40439253
Cortical Thickness Changes in Migraine Patients Treated with Anti-Calcitonin Gene-Related Peptide Monoclonal Antibodies: A Prospective Age- and Sex-Matched Controlled Study., PMID:40426977
The Effect of Fremanezumab on Pain in Patients with Complex Regional Pain Syndrome: Study Protocol of a Randomized, Double-Blind, Proof-of-Concept, Placebo-Controlled Trial., PMID:40426639
Calcitonin gene-related peptide-targeted therapies for migraine., PMID:40343132
Efficacy of monoclonal antibodies against CGRP in migraine patients with fibromyalgia comorbidity: a retrospective monocentric observational study., PMID:40329175
Fremanezumab for the Treatment of Patients With Migraine and Comorbid Major Depressive Disorder: The UNITE Randomized Clinical Trial., PMID:40323613
Increased infection risk in patients on preventive CGRP-targeting therapies- a meta-analysis and clinical effect assessment., PMID:40281424
Efficacy of anti-calcitonin gene-related peptide monoclonal antibodies in hemiplegic migraine: a case report and review of literature., PMID:40264646
Identifying signals of disproportionate reporting for calcitonin gene-related peptide inhibitors: real-world evidence from the FDA adverse event reporting system., PMID:40261259
Migraine treatment with CGRP monoclonal antibodies: patient evaluation of a mandatory treatment holiday in Belgium., PMID:40210844
Predictors of Response to Treatment With Anti-calcitonin Gene-Related Peptide (CGRP) Antibodies in Real-World Patients With Episodic Migraine: A Two- and Four-Month Prospective Study., PMID:40206906
Dual biological treatments in immune-mediated disorders: a single center experience., PMID:40200173
A National Cross-Sectional Survey on Real-World Experiences of Calcitonin Gene-Related Peptide (CGRP) Monoclonal Antibody Use in Adults with Migraine in Finland., PMID:40189729
Health care resource utilization and direct costs incurred over 12 months by patients with migraine initiating self-injectable calcitonin gene-related peptide monoclonal antibodies: A US real-world study., PMID:40152794
Patient satisfaction with calcitonin gene-related peptide monoclonal antibodies in migraine: A multicenter prospective cohort study., PMID:40135519
Fremanezumab for the Treatment of Migraine Complicated by Medication Overuse: A Systematic Review., PMID:40121372
CROATIAN GUIDELINES FOR SPECIFIC PREVENTIVE TREATMENT OF MIGRAINE WITH MONOCLONAL ANTIBODIES TARGETING CALCITONIN GENE-RELATED PEPTIDE (CGRP) (EPTINEZUMAB, FREMANEZUMAB, AND GALCANEZUMAB) OR THE CGRP RECEPTOR (ERENUMAB)., PMID:40104234
Efficacy and continuability of 675 mg fremanezumab administration over 2 years., PMID:40069630
Treatment Patterns and Healthcare Resource Utilization by Gender and Migraine Frequency in Adult Patients Receiving Galcanezumab Versus Standard of Care Preventive Medications Over 24 months: A United States Retrospective Claims Study., PMID:40046564
Real-World Lessons with Fremanezumab as the Third Available CGRP Monoclonal Antibody in a Third-Level Hospital: Focus on the Factors Predicting Response., PMID:40004586
Review: An Update on CGRP Monoclonal Antibodies for the Preventive Treatment of Episodic Migraine., PMID:39998706
Health Technology Assessment: Evaluation of 8 CGRP-Targeted Therapy Drugs for the Treatment of Migraine., PMID:39991088
The anti-CGRP mAb Fremanezumab reverts the anti-inflammatory effects of CGRP in vitro but does not alter disease evolution in a mouse model of progressive multiple sclerosis., PMID:39988092
The effect of calcitonin gene-related peptide monoclonal antibodies on restless legs syndrome in patients with migraine., PMID:39972460
Physical activity as a predictor of fremanezumab response in chronic migraine - the Phy-Fre-Mig study., PMID:39953381
Prevention of Episodic Migraine Headache Using Pharmacologic Treatments in Outpatient Settings: A Clinical Guideline From the American College of Physicians., PMID:39899861
Switching between anti-CGRP monoclonal antibodies in migraine prophylaxis., PMID:39884968
Pharmacological differences and switching among anti-CGRP monoclonal antibodies: A narrative review., PMID:39825578
Two Cases of Ischemic Complications in Abdominoplasty After Use of a New Biologic Migraine Medication., PMID:39816898
US Real-World Effectiveness, Tolerability, and Healthcare Resource Utilization After Addition of Fremanezumab for Preventive Treatment in Patients Using Gepants for Acute Treatment of Migraine: Results From a Retrospective Chart Review., PMID:39775579
Could CGRP mAbs for migraine trigger rheumatoid arthritis? Insights from a case report., PMID:39754737
Comparison of Early-onset Efficacy of Anti-calcitonin Gene-related Peptide Monoclonal Antibodies for Patients with Migraine in Real-world Clinical Practice: Study Protocol for an Exploratory Clinical Trial., PMID:39662907
One-step ultra-rapid immunoassay of calcitonin gene-related peptide for migraine diagnosis., PMID:39608279
Switching from ligand to receptor anti-calcitonin gene-related peptide (CGRP) antibodies or vice versa in non-responders: A controlled cohort study., PMID:39607215
Benefit-risk assessment based on number needed to treat and number needed to harm: Atogepant vs. calcitonin gene-related peptide monoclonal antibodies., PMID:39558612
Crowdsourcing Adverse Events Associated With Monoclonal Antibodies Targeting Calcitonin Gene-Related Peptide Signaling for Migraine Prevention: Natural Language Processing Analysis of Social Media., PMID:39515814
Efficacy of eptinezumab in non-responders to subcutaneous monoclonal antibodies against CGRP and the CGRP receptor: A retrospective cohort study., PMID:39469839
Onabotulinumtoxin A for the Treatment of Post-Traumatic Headache: Is It Better than Anti-CGRP Antibodies?, PMID:39453203
Hypersensitivity to the CGRP Inhibitor Monoclonal Antibodies Galcanezumab, Erenumab, and Fremanezumab With Tolerance to Eptinezumab., PMID:39441051
Efficacy and Tolerability of Anti-CGRP Monoclonal Antibodies in Patients Aged ≥ 65 Years With Daily or Nondaily Migraine., PMID:39399553
Comparative efficacy and safety of different pharmacological therapies to medication overuse headache: a network meta-analysis., PMID:39375607
Preventive drug treatments for adults with chronic migraine: a systematic review with economic modelling., PMID:39365169
Calcitonin Gene-Related Peptide Monoclonal Antibodies: Key Lessons from Real-World Evidence., PMID:39335442
Effectiveness and Tolerability of Anti-Calcitonin Gene-Related Peptide Therapy for Migraine and Other Chronic Headaches in Adolescents and Young Adults: A Retrospective Study in the USA., PMID:39335375
The influence of pharmacodynamics and pharmacokinetics on the antimigraine efficacy and safety of novel anti-CGRPergic pharmacotherapies: a narrative review., PMID:39319681
Case series on monoclonal antibodies targeting calcitonin gene-related peptide in migraine patients during pregnancy: Enhancing safety data., PMID:39314064