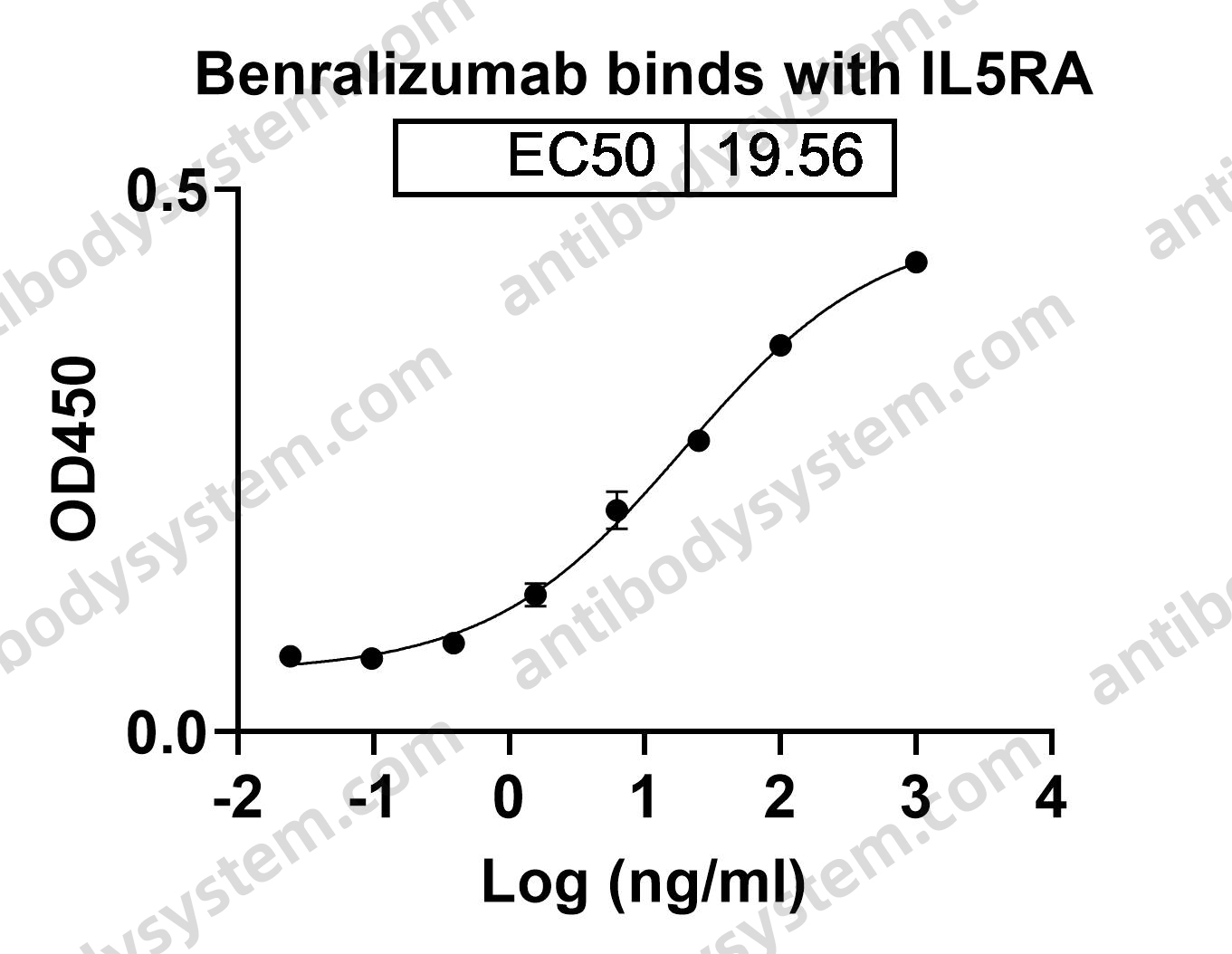
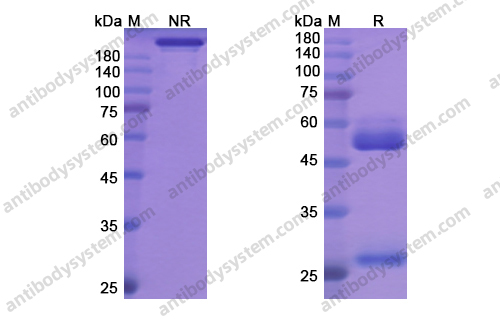
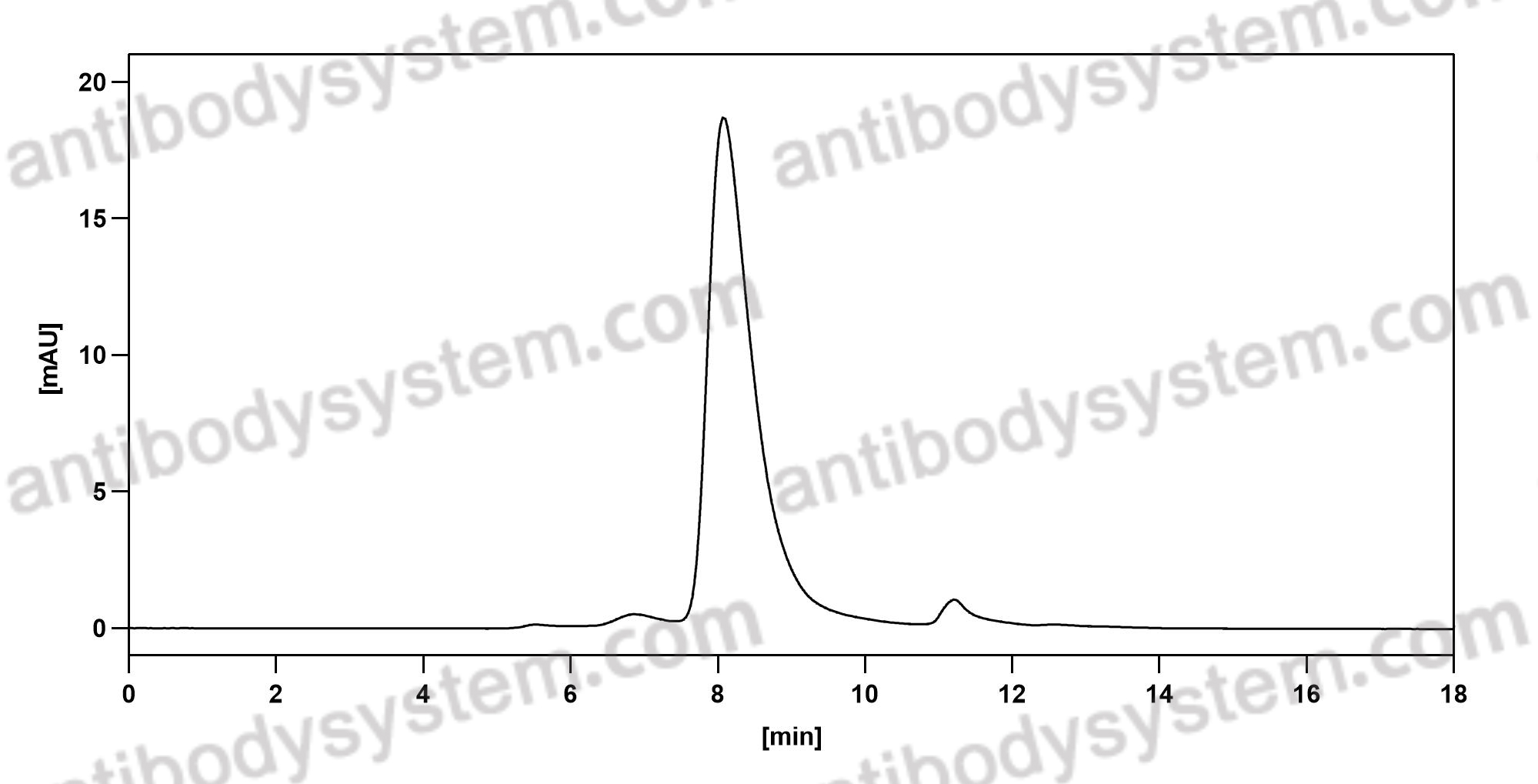
Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma, PMID: 28530840
Benralizumab for the Prevention of COPD Exacerbations, PMID: 31112385
Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma, PMID: 32034960
Real-World Effectiveness of Benralizumab in Severe Eosinophilic Asthma, PMID: 32882249
Benralizumab for PDGFRA-Negative Hypereosinophilic Syndrome, PMID: 30943337
Benralizumab for asthma, PMID: 30410214
Benralizumab: an updated treatment of eosinophilic asthma, PMID: 32133878
Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β 2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial, PMID: 27609408
Benralizumab for Chronic Spontaneous Urticaria, PMID: 32997916
Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial, PMID: 27609406
Benralizumab: A New Approach for the Treatment of Severe Eosinophilic Asthma, PMID: 31017107
Asthma exacerbations on benralizumab are largely non-eosinophilic, PMID: 32767484
Benralizumab as a Steroid-Sparing Treatment Option in Eosinophilic Granulomatosis with Polyangiitis, PMID: 33065367
Benralizumab: First Global Approval, PMID: 29464664
Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial, PMID: 30416083
Benralizumab, PMID: 31643952
Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial, PMID: 33357499
Safety of Eosinophil-Depleting Therapy for Severe, Eosinophilic Asthma: Focus on Benralizumab, PMID: 32242310
Efficacy of benralizumab for patients with severe eosinophilic asthma: a retrospective, real-life study, PMID: 32746787
Efficacy and safety of treatment with biologicals (benralizumab, dupilumab and omalizumab) for severe allergic asthma: A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma, PMID: 32064642
Benralizumab, PMID: 30000011
Benralizumab after sub-optimal response to mepolizumab in severe eosinophilic asthma, PMID: 33300186
Benralizumab efficacy for patients with fixed airflow obstruction and severe, uncontrolled eosinophilic asthma, PMID: 31626906
Benralizumab for the treatment of asthma, PMID: 28379047
Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia, PMID: 23866823
Ability of Serum IgE Concentration to Predict Exacerbation Risk and Benralizumab Efficacy for Patients with Severe Eosinophilic Asthma, PMID: 31836949
Benralizumab for the treatment of asthma, PMID: 29517082
Corticosteroid tapering with benralizumab treatment for eosinophilic asthma: PONENTE Trial, PMID: 31579676
Two-year integrated steroid-sparing analysis and safety of benralizumab for severe asthma, PMID: 31859541
Benralizumab in Real Life, PMID: 32573458
Blood Eosinophil Depletion with Mepolizumab, Benralizumab, and Prednisolone in Eosinophilic Asthma, PMID: 32584603
Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: a systematic review, PMID: 30309978
Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma, PMID: 30139780
Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies, PMID: 28919200
Mabs for treating asthma: omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab, PMID: 32401603
Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study, PMID: 25306557
Benralizumab: an anti-IL-5 receptor α monoclonal antibody in the treatment of asthma, PMID: 29359607
Benralizumab, an add-on treatment for severe eosinophilic asthma: evaluation of exacerbations, emergency department visits, lung function, and oral corticosteroid use, PMID: 30425502
Benralizumab as a potential treatment of asthma, PMID: 28406319
Efficacy and steroid-sparing effect of benralizumab: has it an advantage over its competitors?, PMID: 31024635
Modulation of blood inflammatory markers by benralizumab in patients with eosinophilic airway diseases, PMID: 30658649
The relationship between Feno and effectiveness of mepolizumab and benralizumab in severe eosinophilic asthma, PMID: 33486140
Benralizumab in the treatment of severe asthma: design, development and potential place in therapy, PMID: 29606855
Real-life experience with benralizumab during 6 months, PMID: 32600318
Benralizumab: From the Basic Mechanism of Action to the Potential Use in the Biological Therapy of Severe Eosinophilic Asthma, PMID: 29862274
Rapid effect of benralizumab for severe asthma with chronic rhinosinusitis with nasal polyps, PMID: 33039667
Benralizumab for the add-on maintenance treatment of patients with severe asthma aged 12 years and older with an eosinophilic phenotype, PMID: 29972739
Benralizumab as initial treatment for chronic eosinophilic pneumonia, PMID: 32807690
Benralizumab strongly reduces blood basophils in severe eosinophilic asthma, PMID: 32762056
Anti-IL5 therapies for asthma, PMID: 28933516
Cost-effectiveness of mepolizumab vs anti-interleukin-5/5r biologic therapies for the treatment of adults with severe asthma with an eosinophilic phenotype: a Chilean healthcare system perspective., PMID:40528625
Corrigendum to "Effectiveness of benralizumab in the Tokyo Asthma Study (TOAST): A real-world prospective interventional trial" [Allergol Int 74 (2025) 274-82]., PMID:40517040
ICS use trajectories in severe asthma patients on benralizumab: real-life data from 3-years follow-up., PMID:40513966
Aspirin-Exacerbated Respiratory Disease in the era of biologics., PMID:40490219
Advancements in Biologic Therapies for Pediatric Asthma: Emerging Therapies and Future Directions., PMID:40486460
Benralizumab treatment and 3-dimensional bronchial tree changes in chronic eosinophilic pneumonia., PMID:40476092
Development of Eosinophilic Granulomatosis With Polyangiitis Despite Anti-Interleukin-5 Receptor Therapy: The First Case of Bilateral Central Retinal Artery Occlusion During Benralizumab Treatment., PMID:40461442
Two unique cases of eosinophilic granulomatosis with polyangiitis in childhood treated with anti-interleukin-5 therapy: infantile-onset and submandibular salivary gland involvement., PMID:40457436
Efficacy of Benralizumab in Reducing FeNO in Severe Eosinophilic Asthma: The Role of CRSwNP., PMID:40454227
Advancing Remission in Severe Asthma With Benralizumab: Latest Findings, Current Perspectives and Future Direction., PMID:40444568
Hypereosinophilia: clinical and therapeutic approach in 2025., PMID:40396537
Benralizumab for acute thromboembolism in hypereosinophilic syndrome: a case report., PMID:40382631
Long-Term Longitudinal Analysis of Pulmonary Function Before and After Biological Therapy in Severe Asthma., PMID:40373751
Biologic Therapies for Severe Asthma: Current Insights and Future Directions., PMID:40364184
Early and Sustained Clinical Benefits of Benralizumab in Severe Eosinophilic Asthma: Findings from the ORBE II Study., PMID:40364043
Requalification of patients with severe asthma for biological therapy-Practical 'ReQuaBi' rate decision scheme based on the analytical model., PMID:40348613
High local type-2 inflammation is linked to response in severe asthma treated with anti-Interleukin-5 receptor., PMID:40345261
Reappraisal of Biologic Efficacy from Phase 3 Trials in Refractory Chronic Rhinosinusitis and Nasal Polyps., PMID:40340024
Comparative Insights on IL-5 Targeting with Mepolizumab and Benralizumab: Enhancing EGPA Treatment Strategies., PMID:40305320
Pathway to Remission in Severe Asthma: Clinical Effectiveness and Key Predictors of Success with Benralizumab Therapy: A Real-Life Study., PMID:40299478
Long-term efficacy and safety of different biologics in treatment of chronic rhinosinusitis with nasal polyps: A network meta-analysis., PMID:40286594
Biologic Agents in Idiopathic Hypereosinophilic Syndrome., PMID:40283978
New Therapeutic Challenges in Pediatric Gastroenterology: A Narrative Review., PMID:40281872
Efficacy of Biologics in Reducing Exacerbations Requiring Hospitalization or an Emergency Department Visit in Patients with Moderate or Severe, Uncontrolled Asthma., PMID:40261563
XALOC-1: Clinical Remission Over 2 Years With Benralizumab in Severe Eosinophilic Asthma., PMID:40258512
Exploring a novel approach to COPD treatment: benralizumab effect in an ex vivo 3-D model., PMID:40254172
Circulating Mast Cell Progenitors Are Depleted by Benralizumab in Severe Asthma., PMID:40251899
Comparative Effectiveness of Dupilumab Versus Mepolizumab and Benralizumab in Asthma: A Multinational Retrospective Cohort Study., PMID:40250559
Effectiveness of tezepelumab in preventing relapse of eosinophilic granulomatosis with polyangiitis: A case report., PMID:40239417
Treatment of allergic bronchopulmonary aspergillosis with biologics., PMID:40226607
Utility of the Basophil Reactivity Test in the Clinical Management of People with Severe Uncontrolled Asthma., PMID:40224171
Real-World Evidence of Administration of Biologic Agents in Patients with Severe Asthma: An Analysis of the Respiratory Department of University Hospital of Patras Asthma Registry., PMID:40217624
Combining Dual Biologics Therapy for Severe Asthma: A Series of Ten Cases., PMID:40206518
Benralizumab and the integrated management of co-morbid severe eosinophilic asthma with chronic rhinosinusitis with nasal polyps., PMID:40200389
Dual biological treatments in immune-mediated disorders: a single center experience., PMID:40200173
Effect of benralizumab treatment on the airway microbiome in COPD., PMID:40196715
Long-term, real-world effectiveness of biologics for severe uncontrolled asthma: The PROSPECT study., PMID:40186961
Head-to-Head Effectiveness Comparison of Biological Therapies in Patients With Mixed Eosinophilic and Allergic Severe Asthma., PMID:40185202
Durability of benralizumab effectiveness in severe eosinophilic asthma patients with and without chronic rhinosinusitis with nasal polyps: a post hoc analysis from the ANANKE study., PMID:40181809
Pulmonology: What You May Have Missed in 2024., PMID:40163874
Benralizumab for the treatment of chronic spontaneous urticaria: a plain language summary of the ARROYO study., PMID:40159728
Head-To-Head Comparison of Biologic Efficacy in Asthma: What Have We Learned?, PMID:40156481
Severe Asthma and Active SARS-CoV-2 Infection: Insights into Biologics., PMID:40149651
Mepolizumab versus benralizumab for eosinophilic granulomatosis with polyangiitis (EGPA): A European real-life retrospective comparative study., PMID:40147217
Long-Term Clinical Remission on Benralizumab Treatment in Severe Eosinophilic Asthma: A Four-Year Real-Life Study., PMID:40142883
Clinical efficacy and mechanisms of biologics for chronic rhinosinusitis with nasal polyps., PMID:40132672
Long-Term Efficacy and Safety of Benralizumab Treatment for PDGFRA-Negative Hypereosinophilic Syndrome., PMID:40120806
Adverse effects of biologics used to treat asthma., PMID:40099886
Effect of Biologics against Interleukin-5 Administered to Patients with Eosinophilic Gastroenteritis1., PMID:40090714
Real-World Effectiveness of Biologic Therapy in Allergic Bronchopulmonary Aspergillosis., PMID:40088970