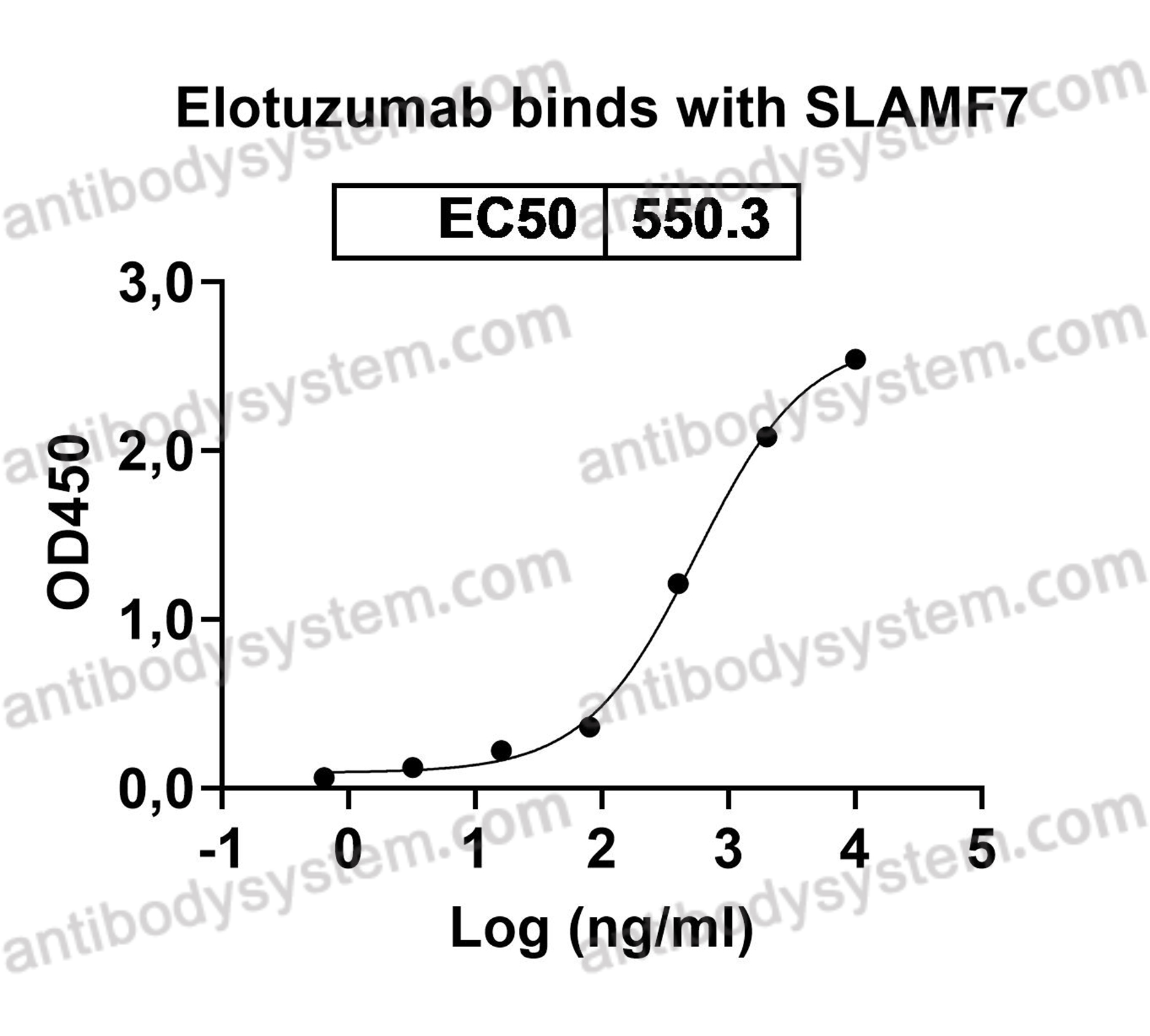
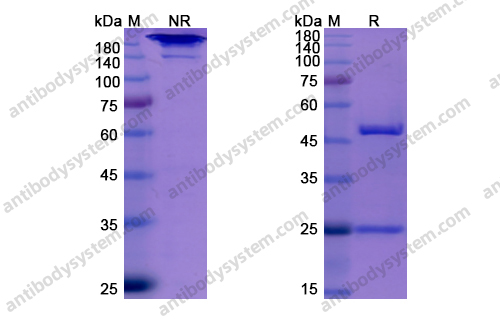
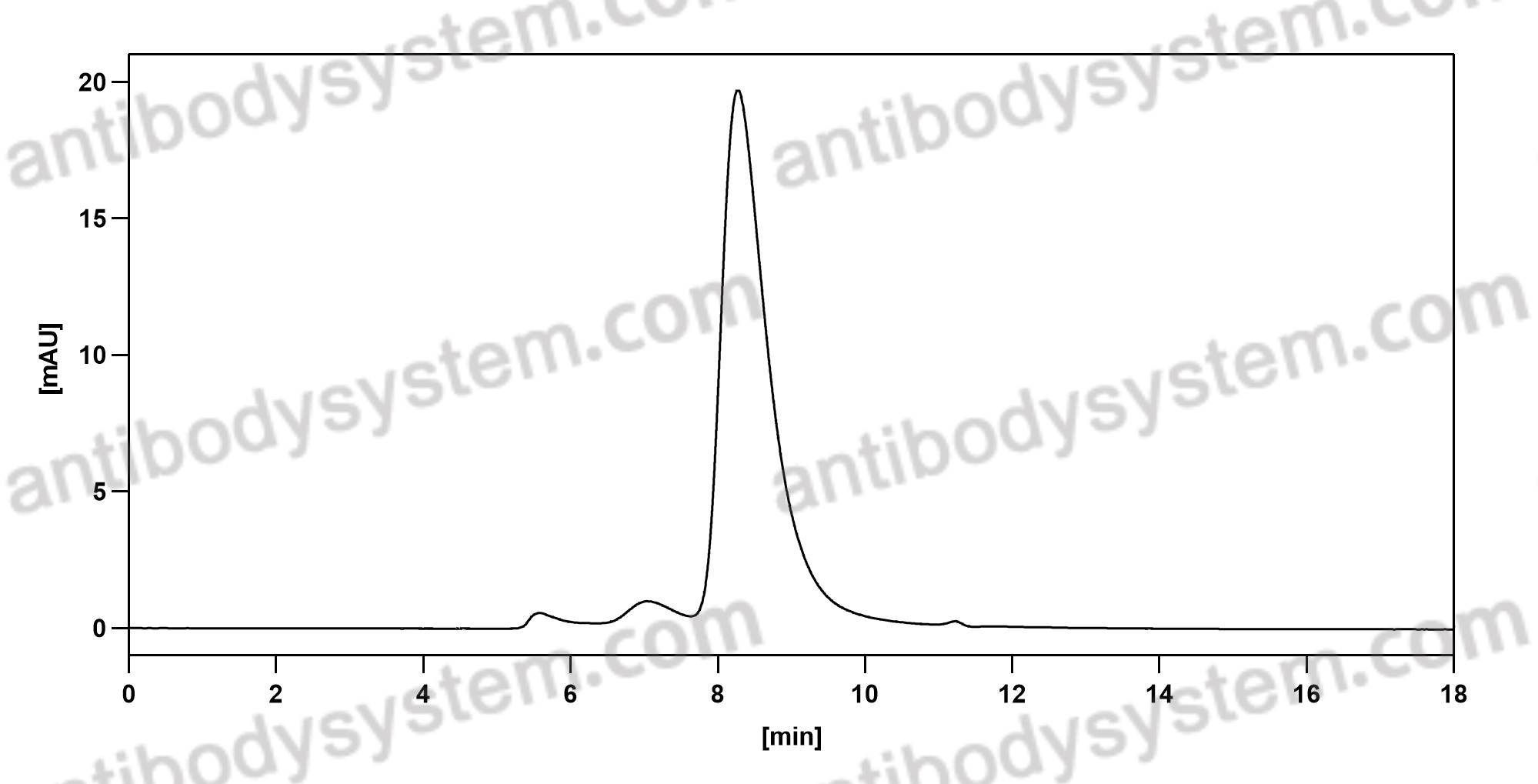
Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma, PMID: 30403938
Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma, PMID: 26035255
The Clinical Pharmacology of Elotuzumab, PMID: 28779463
Elotuzumab, PMID: 31644037
Elotuzumab: A Review in Relapsed and/or Refractory Multiple Myeloma, PMID: 30232695
Mechanisms of NK Cell Activation and Clinical Activity of the Therapeutic SLAMF7 Antibody, Elotuzumab in Multiple Myeloma, PMID: 30455698
Elotuzumab, PMID: 29999843
Bortezomib, lenalidomide, and dexamethasone with or without elotuzumab in patients with untreated, high-risk multiple myeloma (SWOG-1211): primary analysis of a randomised, phase 2 trial, PMID: 33357482
The role of elotuzumab in the treatment of relapsed or refractory multiple myeloma, PMID: 29317395
Elotuzumab, lenalidomide, and dexamethasone in RRMM: final overall survival results from the phase 3 randomized ELOQUENT-2 study, PMID: 32887873
Elotuzumab in combination with pomalidomide and dexamethasone for the treatment of multiple myeloma, PMID: 31679403
Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4-year follow-up and analysis of relative progression-free survival from the randomized ELOQUENT-2 trial, PMID: 30204239
Elotuzumab: First Global Approval, PMID: 26809244
Elotuzumab for the Treatment of Relapsed or Refractory Multiple Myeloma, with Special Reference to its Modes of Action and SLAMF7 Signaling, PMID: 29531651
Elotuzumab as a novel anti-myeloma immunotherapy, PMID: 28604269
Elotuzumab: the first approved monoclonal antibody for multiple myeloma treatment, PMID: 27493709
Elotuzumab-based maintenance therapy following autologous stem cell transplant in multiple myeloma deepens post-transplant responses, PMID: 32745939
Development of [ 89 Zr]DFO-elotuzumab for immunoPET imaging of CS1 in multiple myeloma, PMID: 33179150
Elotuzumab for the treatment of multiple myeloma, PMID: 27417553
Update on elotuzumab for the treatment of relapsed/refractory multiple myeloma: patients' selection and perspective, PMID: 31410026
Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM, PMID: 27091875
Efficacy and safety of elotuzumab for the treatment of multiple myeloma, PMID: 28060563
Elotuzumab in multiple myeloma, PMID: 30449660
Multiple Myeloma: Diagnosis and Treatment, PMID: 26763514
Second elotuzumab triplet efficacious in MM, PMID: 30478425
Treatment of multiple myeloma with the immunostimulatory SLAMF7 antibody elotuzumab, PMID: 27695618
Spotlight on elotuzumab in the treatment of multiple myeloma: the evidence to date, PMID: 27785050
Daratumumab, Elotuzumab, and the Development of Therapeutic Monoclonal Antibodies in Multiple Myeloma, PMID: 27806428
Elotuzumab: a novel immune-stimulating therapy to treat multiple myeloma, PMID: 27322214
Elotuzumab: a novel anti-CS1 monoclonal antibody for the treatment of multiple myeloma, PMID: 24151843
Immunotherapy: A New Approach to Treating Multiple Myeloma with Daratumumab and Elotuzumab, PMID: 27083916
Update on elotuzumab, a novel anti-SLAMF7 monoclonal antibody for the treatment of multiple myeloma, PMID: 27533882
Toward further simplification of elotuzumab therapy by subcutaneous administration, PMID: 32656639
Treatment of relapsed and refractory multiple myeloma, PMID: 32719176
Model-Based Determination of Elotuzumab Pharmacokinetics in Japanese Patients With Multiple Myeloma Incorporating Time-Varying M Protein, PMID: 32656777
Treatment of relapsed multiple myeloma: Evidence-based recommendations, PMID: 31500848
Mechanisms of Action and Clinical Development of Elotuzumab, PMID: 29272564
Elotuzumab in the treatment of relapsed and refractory multiple myeloma, PMID: 33478270
Frequent occurrence of hypophosphatemia among multiple myeloma patients treated with elotuzumab: a single clinic retrospective study, PMID: 33237342
Sequence matters: elotuzumab more effective if used before daratumumab, PMID: 31845607
How to Integrate Elotuzumab and Daratumumab Into Therapy for Multiple Myeloma, PMID: 27998219
Managing Infusion Reactions to New Monoclonal Antibodies in Multiple Myeloma: Daratumumab and Elotuzumab, PMID: 29996069
Elotuzumab for the treatment of multiple myeloma, PMID: 24941981
Elotuzumab and daratumumab: emerging new monoclonal antibodies for multiple myeloma, PMID: 24053207
Emerging immunotherapies in multiple myeloma, PMID: 32958461
Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy, PMID: 30147690
Elotuzumab: The First Monoclonal Antibody for the Treatment of Multiple Myeloma, PMID: 29282429
A review discussing elotuzumab and its use in the second-line plus treatment of multiple myeloma, PMID: 29091475
Soluble SLAMF7 is a predictive biomarker for elotuzumab therapy, PMID: 32398792
Elotuzumab-induced interstitial lung disease: the first case report, PMID: 29635485
Daratumumab (anti-CD38)- and elotuzumab (anti-SLAMF7)-based treatments for refractory POEMS syndrome: a single-center case series., PMID:40528367
Global Access to Multiple Myeloma Therapies., PMID:40466014
The efficacy of new drug regimens in treating newly diagnosed high-risk cytogenetic multiple myeloma patients: a systematic literature review and meta-analysis., PMID:40432724
Real world outcomes with elotuzumab-based therapies for patients with relapsed refractory multiple myeloma: a Mayo Clinic experience., PMID:40410136
Using bispecific antibodies in a patient with peritoneal dialysis for relapsed multiple myeloma., PMID:40356496
Comparative Efficacy of Ciltacabtagene Autoleucel Versus Standard-of-Care Treatments for Patients with Previously Treated Relapsed or Refractory Multiple Myeloma: A Matching-Adjusted Indirect Comparison., PMID:40354013
Pulmonary adverse events associated with monoclonal antibodies approved for multiple myeloma: a pharmacovigilance study based on the FDA adverse event reporting system., PMID:40302414
Elotuzumab in Combination With Dose Reduced IMiDs and Dexamethasone for AL Amyloidosis Patients With Relapsed/Refractory Plasma Cell Dyscrasia and Advanced Organ Involvement., PMID:40276975
Prognostic Significance of +1q Alterations in Relapsed/Refractory Multiple Myeloma Treated With Daratumumab-, Elotuzumab-, and Carfilzomib-Based Triplet Regimens: A Multicenter Real-World Analysis of 635 Patients., PMID:40103518
Unveiling cardiovascular and respiratory toxicities with monoclonal antibodies in multiple myeloma: disproportionality analysis from the FDA Adverse Event Reporting System., PMID:40095047
Cost-effectiveness analysis of combination therapies involving novel agents for first/second-relapse patients with multiple myeloma: a Markov model approach with calibration techniques., PMID:40088315
The multifaceted role of CS1 (SLAMF7) in immunoregulation: Implications for cancer therapy and autoimmune disorders., PMID:40073958
Memory-like NK cell differentiation, inhibitory NKG2A blockade, and improved recognition via antibody or CAR engineering combine to enhance NK cell attack against multiple myeloma., PMID:40073259
Comparison of isatuximab-pomalidomide-dexamethasone versus elotuzumab-pomalidomidedexamethasone in relapsed/refractory multiple myeloma patients: a target trial emulation using real-world data., PMID:40045883
Monoclonal Antibodies in Relapsed-Refractory Multiple Myeloma., PMID:40005960
Monoclonal Antibodies and Small-Molecule Inhibitors Associated Osteonecrosis of Jaw: A Retrospective Pharmacovigilance Study., PMID:39922223
Angioimmunoblastic T-cell lymphoma: Characterization of clonal T and B cells and a patient-derived xenograft study of coexisting T- and B-cell proliferation., PMID:39877932
Correction to Elotuzumab Plus Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma: Extended Follow-Up of a Multicenter, Retrospective Real-World Experience With 321 Cases Outside of Controlled Clinical Trials., PMID:39846436
Outcomes and prognostic indicators in daratumumab-refractory multiple myeloma: a multicenter real-world study of elotuzumab, pomalidomide, and dexamethasone in 247 patients., PMID:39778329
Advancements in the Treatment of Multiple Myeloma., PMID:39744254
Elotuzumab in combination with pomalidomide, bortezomib, and dexamethasone in relapsed and refractory multiple myeloma., PMID:39626297
Elotuzumab-mediated ADCC with Th1-like Vγ9Vδ2 T cells to disrupt myeloma-osteoclast interaction., PMID:39557586
Novel Drug Combinations and Donor Lymphocyte Infusions Allow Prolonged Disease Control in Multiple Myeloma Patients Relapsing after Allogeneic Transplantation., PMID:39505212
In vitro testing of drug response in primary multiple myeloma cells using a microwell-based technology., PMID:39486120
Neuropsychiatric Adverse Events with Monoclonal Antibodies Approved for Multiple Myeloma: An Analysis from the FDA Adverse Event Reporting System., PMID:39458907
The Real-World Outcomes of Relapsed/Refractory Multiple Myeloma Treated with Elotuzumab, Pomalidomide, and Dexamethasone., PMID:39449301
Addition of Elotuzumab to Backbone Treatment Regimens for Multiple Myeloma: An Updated Meta-Analysis of Randomized Clinical Trials., PMID:39414558
Cardiovascular adverse events associated with targeted therapies for multiple myeloma: a pharmacovigilance study., PMID:39391316
Dexamethasone dose intensity does not impact outcomes in newly diagnosed multiple myeloma: a secondary SWOG analysis., PMID:39321347
Phase II trial of elotuzumab with pomalidomide and dexamethasone for daratumumab-refractory multiple myeloma., PMID:39237502
Disseminated Nontuberculous Mycobacterium Infection During Treatment of Multiple Myeloma: A Case Report and Review of the Literature., PMID:39231663
From Triple- to Penta-Exposed Multiple Myeloma: A Real-World Study in a Medicare Population., PMID:39097860
Post-ASCT consolidation with elotuzumab, lenalidomide, and dexamethasone or elotuzumab, pomalidomide, and dexamethasone in high-risk and ultra-high-risk multiple myeloma: a retrospective single-center study., PMID:39082756
Descriptive Analysis of Adverse Events Reported for New Multiple Myeloma Medications Using FDA Adverse Event Reporting System (FAERS) Databases from 2015 to 2022., PMID:39065666
Improvement of daratumumab- or elotuzumab-mediated NK cell activity by the bi-specific 4-1BB agonist, DARPin α-FAPx4-1BB: A preclinical study in multiple myeloma., PMID:38850654
Nivolumab, Pomalidomide, and Elotuzumab Combination Regimens for Treatment of Relapsed and Refractory Multiple Myeloma: Results from the Phase 3 CheckMate 602 Study., PMID:38849283
Elotuzumab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of a multicenter, retrospective real-world experience with 321 cases outside of controlled clinical trials., PMID:38818978
CS1 Expression Pattern in NK Cells and Correlated Factors in Plasma Cell dyscrasias: Implications for Elotuzumab Therapy and CAR-T Efficacy., PMID:38706917
Cytomegalovirus Reactivation during Elotuzumab Therapy in Patients with Multiple Myeloma., PMID:38657575
Daratumumab‑resistant multiple myeloma with extramedullary disease successfully treated with combination elotuzumab, pomalidomide and dexamethasone: A case report., PMID:38638843
Therapeutic progress in relapsed/refractory multiple myeloma., PMID:38609727
Phase 1 study combining elotuzumab with autologous stem cell transplant and lenalidomide for multiple myeloma., PMID:38609316
Characteristics of isatuximab-derived interference in serum protein electrophoresis and immunofixation, and an absence of sustained in vivo interference due to belantamab mafodotin and denosumab., PMID:38565341
Comparative effectiveness of lenalidomide/dexamethasone-based triplet regimens for treatment of relapsed and/or refractory multiple myeloma in the United States: An analysis of real-world electronic health records data., PMID:38547609
Safety and Effectiveness of Elotuzumab in Japanese Patients with Relapsed/Refractory Multiple Myeloma: A Post-marketing Surveillance Study., PMID:38494720
Efficacy of elotuzumab for multiple myeloma deteriorates after daratumumab: a multicenter retrospective study., PMID:38492020
Elotuzumab Enhances CD16-Independent NK Cell-Mediated Cytotoxicity against Myeloma Cells by Upregulating Several NK Cell-Enhancing Genes., PMID:38444839
NK and T-lymphocyte Kinetics Predict Outcome in Myeloma Patients Treated With Elotuzumab, Lenalidomide Plus Dexamethasone., PMID:38434915
Health-related quality of life in patients with triple-class exposed relapsed and refractory multiple myeloma treated with idecabtagene vicleucel or standard regimens: patient-reported outcomes from the phase 3, randomised, open-label KarMMa-3 clinical trial., PMID:38423700
Elotuzumab: no additional effect in patients with newly diagnosed multiple myeloma., PMID:38302226