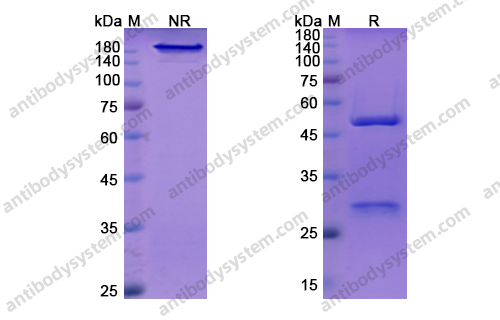
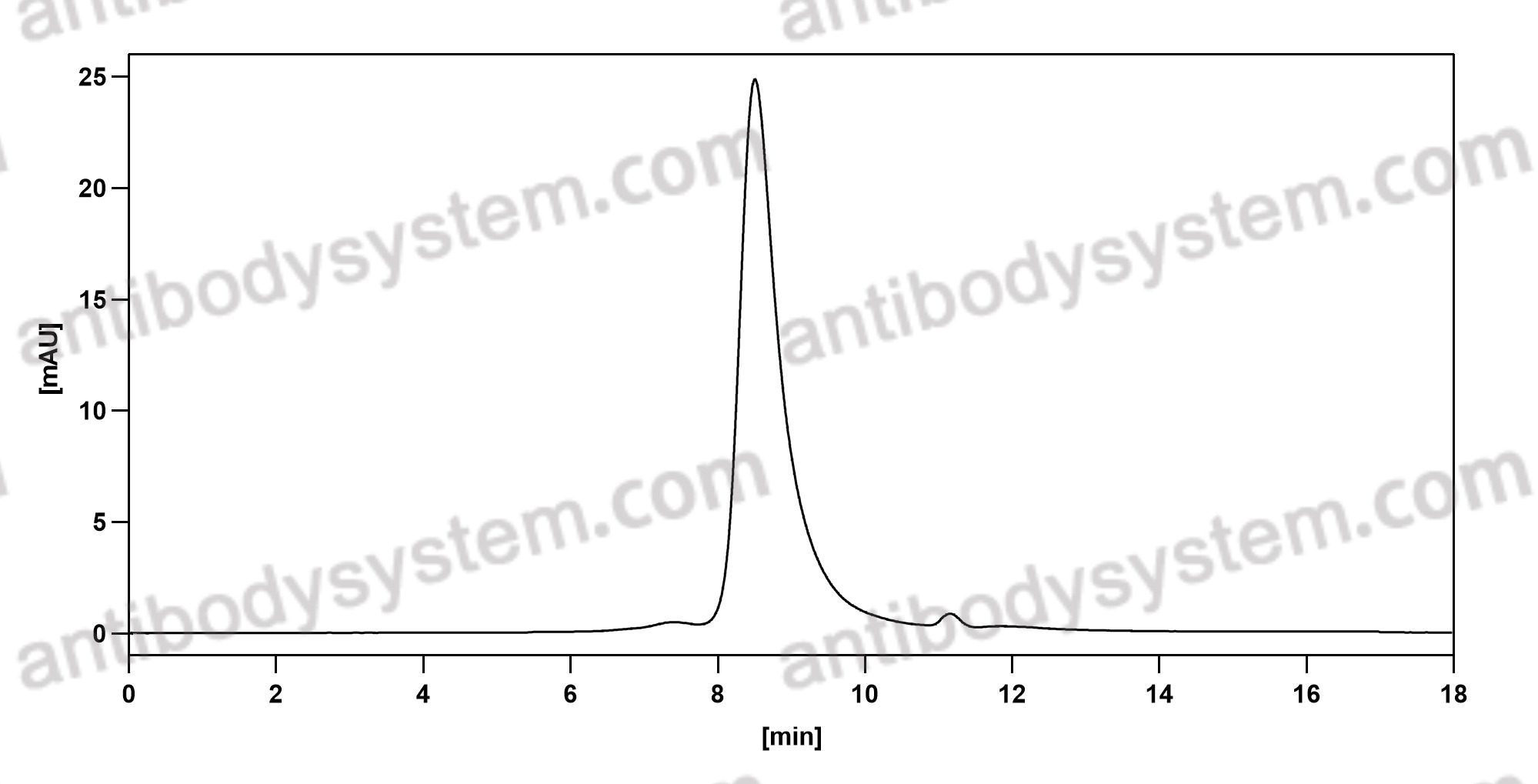
Vedolizumab as induction and maintenance therapy for ulcerative colitis, PMID: 23964932
Long-term safety of vedolizumab for inflammatory bowel disease, PMID: 32876349
Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients With Ulcerative Colitis, PMID: 31470005
Vedolizumab as induction and maintenance therapy for Crohn's disease, PMID: 23964933
The safety of vedolizumab for the treatment of ulcerative colitis, PMID: 28276855
Safety of New Biologics (Vedolizumab and Ustekinumab) and Small Molecules (Tofacitinib) During Pregnancy: A Review, PMID: 32562207
The safety of vedolizumab for ulcerative colitis and Crohn's disease, PMID: 26893500
Long-term Efficacy of Vedolizumab for Crohn's Disease, PMID: 27683798
Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview, PMID: 31064370
Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis, PMID: 28204866
A Review of the Clinical Pharmacokinetics, Pharmacodynamics, and Immunogenicity of Vedolizumab, PMID: 28523450
Vedolizumab Therapy in Severe Pediatric Inflammatory Bowel Disease, PMID: 27598742
Endoscopic, Radiologic, and Histologic Healing With Vedolizumab in Patients With Active Crohn's Disease, PMID: 31279871
Comparative safety and effectiveness of vedolizumab to tumour necrosis factor antagonist therapy for Crohn's disease, PMID: 32656800
Long-term Efficacy of Vedolizumab for Ulcerative Colitis, PMID: 27683800
Therapeutic Drug Monitoring With Ustekinumab and Vedolizumab in Inflammatory Bowel Disease, PMID: 29788272
Vedolizumab Induces Endoscopic and Histologic Remission in Patients With Crohn's Disease, PMID: 31175865
Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease, PMID: 29430672
Effects of Vedolizumab Therapy on Extraintestinal Manifestations in Inflammatory Bowel Disease, PMID: 29484571
Vedolizumab in the Perioperative Management of Inflammatory Bowel Disease, PMID: 30914021
Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn's disease patients with prior failure to anti-TNF treatment, PMID: 32441396
Safety and Effectiveness of Vedolizumab in Patients with Steroid-Refractory Gastrointestinal Acute Graft-versus-Host Disease: A Retrospective Record Review, PMID: 30468919
Vedolizumab in Japanese patients with ulcerative colitis: A Phase 3, randomized, double-blind, placebo-controlled study, PMID: 30807613
Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients With Inflammatory Bowel Disease: From Basic Science to Clinical Practice, PMID: 31777929
Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease, PMID: 29730603
Vedolizumab Treatment in Extra-Intestinal Manifestations in Inflammatory Bowel Disease: A Systematic Review, PMID: 31076751
Vedolizumab and etrolizumab for ulcerative colitis: twins or simple cousins?, PMID: 31951748
An Overview of the Mechanism of Action of the Monoclonal Antibody Vedolizumab, PMID: 27252400
Vedolizumab for perianal Crohn's disease: a multicentre cohort study in 151 patients, PMID: 32080886
Vedolizumab treatment persistence and safety in a 2-year data analysis of an extended access programme, PMID: 33210333
Vedolizumab for the treatment of Crohn's disease, PMID: 29406811
Vedolizumab use after failure of TNF-α antagonists in children and adolescents with inflammatory bowel disease, PMID: 30219028
Rapid Response to Vedolizumab Therapy in Biologic-Naive Patients With Inflammatory Bowel Diseases, PMID: 29857145
Vedolizumab use is not associated with increased malignancy incidence: GEMINI LTS study results and post-marketing data, PMID: 31747086
The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn's disease refractory to anti-tumour necrosis factor, PMID: 32249966
A product review of vedolizumab in inflammatory bowel disease, PMID: 30897022
Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: a multicentre cohort study, PMID: 33345331
Efficacy of Vedolizumab in Fistulising Crohn's Disease: Exploratory Analyses of Data from GEMINI 2, PMID: 29471381
Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study, PMID: 30518410
Development and Validation of Clinical Scoring Tool to Predict Outcomes of Treatment With Vedolizumab in Patients With Ulcerative Colitis, PMID: 32062041
Should we use vedolizumab as mono or combo therapy in ulcerative colitis?, PMID: 30060935
Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn's disease, PMID: 25996351
Infliximab, adalimumab and vedolizumab concentrations across pregnancy and vedolizumab concentrations in infants following intrauterine exposure, PMID: 32981127
Drug survival of anti-TNF agents compared with vedolizumab as a second-line biological treatment in inflammatory bowel disease: results from nationwide Swedish registers, PMID: 33340426
Benefit-Risk Assessment of Vedolizumab in the Treatment of Crohn's Disease and Ulcerative Colitis, PMID: 30830573
Therapeutic drug monitoring with vedolizumab in inflammatory bowel disease, PMID: 31646853
Vedolizumab for the treatment of inflammatory bowel diseases: from symptomatic control to mucosal healing, PMID: 30860423
Loss of Response to Vedolizumab and Ability of Dose Intensification to Restore Response in Patients With Crohn's Disease or Ulcerative Colitis: A Systematic Review and Meta-analysis, PMID: 29935327
Comorbidity, not patient age, is associated with impaired safety outcomes in vedolizumab- and ustekinumab-treated patients with inflammatory bowel disease-a prospective multicentre cohort study, PMID: 32901983
Efficacy and Safety of Vedolizumab in Ulcerative Colitis and Crohn's Disease Patients Stratified by Age, PMID: 28070861
Influence of Pharmacokinetic-Pharmacodynamic Failure Mechanisms on Outcomes in Biologic Therapy Sequencing in Inflammatory Bowel Disease: A Retrospective Cohort Study., PMID:40531271
Hepatotoxicity associated with vedolizumab: case report in a patient with ulcerative colitis., PMID:40530502
Clinical features, pathogenesis, and treatment of inflammatory bowel disease-associated spondyloarthritis., PMID:40527403
A Guide to the Patient-Centric Use of Vedolizumab for Crohn's Disease., PMID:40512051
Serum proteomic and metabolomic analyses from patients with IBD identify biological pathways associated with treatment success with anti-integrin therapy., PMID:40509637
Birth outcomes in women who have taken vedolizumab in pregnancy: results from the Vedolizumab Pregnancy Exposure Registry., PMID:40498121
The Effectiveness of second-and-third line biologics in perianal Crohn's disease - a multicenter propensity score-matched study., PMID:40490896
Upadacitinib and vedolizumab combination therapy for the management of refractory ulcerative colitis and Crohn's disease., PMID:40490017
Efficacy and Safety of Vedolizumab with Glucocorticoids vs Infliximabin Severe Ulcerative Colitis: A Retrospective Study., PMID:40487981
Treatment sequences, outcomes, healthcare utilization, and costs in patients with inflammatory bowel diseases requiring advanced treatment-real world comparative effectiveness from German claims data., PMID:40481399
Comparative Efficacy of Different Targeted Therapies in Patients With Moderate-to-Severe Ulcerative Colitis: Systematic Review/Network Meta-Analysis and Mechanistic Overview., PMID:40468857
Lack of Efficacy of Concomitant 5-Aminosalicylic Acid With Vedolizumab in Inflammatory Bowel Disease: A Large-Scale Administrative Database Analysis., PMID:40468574
The duration of prior anti-tumor necrosis factor agents is associated with the effectiveness of vedolizumab in patients with ulcerative colitis: a real-world multicenter retrospective study., PMID:40462299
Cancer Incidence in Patients with Ulcerative Colitis Naïve to or Treated with Thiopurine and Targeted Therapies- a cohort study 2007 to 2022 with comparison to the general population., PMID:40455688
Treatment of Autoimmune Enteropathy With Vedolizumab., PMID:40452653
Ultrafast Microdroplet Digestion of Antibodies with Fc-Silencing Mutations., PMID:40438971
Advanced Therapies for Inflammatory Bowel Disease and Risk of Skin Cancer: What's New?, PMID:40427207
The Effects of the Biological Agents Infliximab, Vedolizumab, and Ustekinumab on Intestinal Anastomosis: An Experimental Study in Rats., PMID:40426907
Efficacy and safety of dual-targeted therapy for refractory inflammatory bowel disease: a retrospective case series from three tertiary general hospitals in China., PMID:40421289
Network Meta-Analysis: Efficacy of Biological Therapies and Small Molecules as Maintenance Therapy in Ulcerative Colitis., PMID:40407729
First Description of Simultaneous Sweet Syndrome and Tracheal Stenosis as Extraintestinal Manifestations in a Patient With Highly Active Ulcerative Colitis., PMID:40395659
Marked mucosal lipid shifts in treatment refractory inflammatory bowel disease: a lipidomic study., PMID:40394475
Real-World Effectiveness and Safety of Vedolizumab in Patients ≥ 70 Versus < 70 Years With Ulcerative Colitis: Multicenter Retrospective Study., PMID:40370285
Vedolizumab in inflammatory bowel disease: Real-world outcomes and their prediction with machine learning-the IG-IBD LIVE study., PMID:40360308
Deprescribing 5-Aminosalicytes in Patients with Ulcerative Colitis on Concomitant Advanced Therapy: A Qualitative Analysis., PMID:40356873
Pro-inflammatory macrophages contribute to developing comorbid anxiety-like behaviors through gastrointestinal vagal afferent signaling in experimental colitis mice., PMID:40348137
Second-line strategies after anti-TNF failure in chronically active, moderate-to-severe ulcerative colitis: a retrospective, multicentre cohort study., PMID:40346848
Fatigue Is Strongly Associated with Depressive Symptoms in Patients with Inflammatory Bowel Disease., PMID:40337726
Real-life effectiveness and safety of tofacitinib and vedolizumab as 2nd-line for ulcerative colitis after anti-TNFs: A multicenter cohort IGIBD study (VE2TO-UC)., PMID:40335332
Association between inflammatory bowel disease, current therapies, and cardiovascular events: a review and meta-analysis of data from 2.2 million individuals., PMID:40333172
Intravenous and subcutaneous vedolizumab for moderately to severely active ulcerative colitis in Iran: a model-based cost-effectiveness evaluation., PMID:40329592
The Effectiveness of Medical Therapies for Joint, Skin and Eye Extraintestinal Manifestations in IBD-An Umbrella Review., PMID:40329548
Mirikizumab (Omvoh) - an IL-23 antagonist for Crohn's disease., PMID:40324965
Immunopathological and microbial signatures of inflammatory bowel disease in partial RAG deficiency., PMID:40314722
Clear Cell Renal Carcinoma in an Ulcerative Colitis Patient Under Short-Term Immunosuppressive Therapy: A Case Report., PMID:40310301
The Vulnerability of the Heart During Diarrhea: A Case Report on Pericarditis Linked to Inflammatory Bowel Disease., PMID:40309294
Vedolizumab-steroid combination therapy improves long-term prognosis in patients with ulcerative colitis., PMID:40307703
Author's Reply: "Ustekinumab and vedolizumab: A step forward in managing complex perianal fistulas in Crohn's disease"., PMID:40307165
Rejection and graft vasculopathy secondary to effects of Crohn's disease in a heart-transplanted child., PMID:40306436
Gut-directed therapeutics in inflammatory bowel disease., PMID:40305008
Advanced Therapies in Elderly Patients With Inflammatory Bowel Disease: A Comparative Retrospective Cohort Study in Taiwan., PMID:40303313
Identification of candidate biomarkers and pathways associated with vedolizumab response in T cell populations of IBD patients by WGCNA., PMID:40285783
Role of Gut Microbiota and Metabolomics in Predicting Response to Vedolizumab in Inflammatory Bowel Disease: A Systematic Review., PMID:40284471
New Therapeutic Challenges in Pediatric Gastroenterology: A Narrative Review., PMID:40281872
Post-transplant cyclophosphamide, abatacept, and vedolizumab to prevent GVHD after hematopoietic stem cells transplantation in children with acute leukemia: results of a prospective trial., PMID:40281208
Identification of S100A9 as a target for diagnosis and treatment of Crohn's Disease after Vedolizumab treatment failure., PMID:40280281
Relapsed refractory immune checkpoint inhibitor-induced colitis treated with tacrolimus and maintained with vedolizumab., PMID:40279047
Racial Disparities in Utilization of Medications and Disease Outcomes in Inflammatory Bowel Disease Patients., PMID:40260307
Triple biologic therapy for refractory Crohn's disease., PMID:40251965
Impact of centralised reconstitution of biological medicines on the amount and costs of medicine waste., PMID:40246549