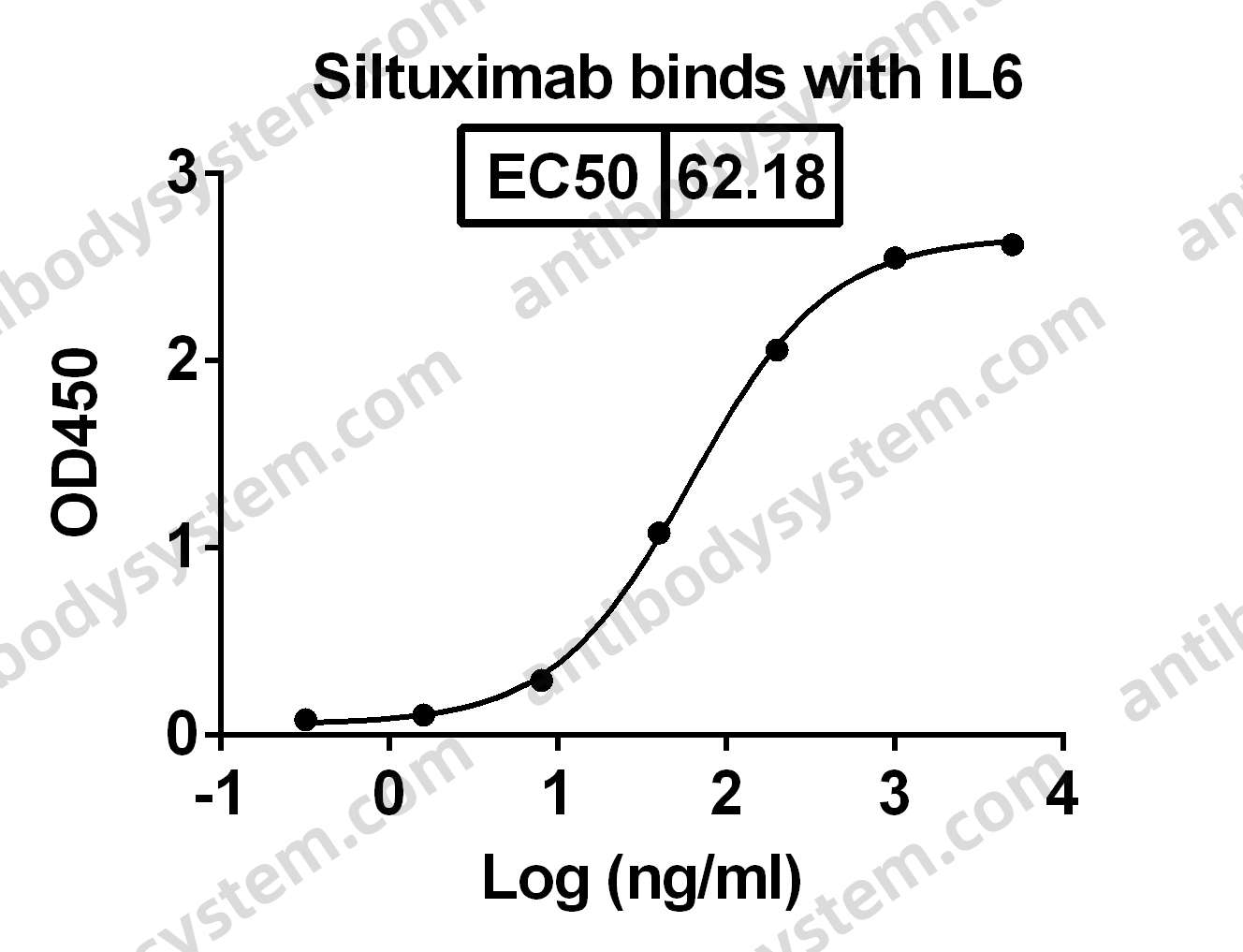
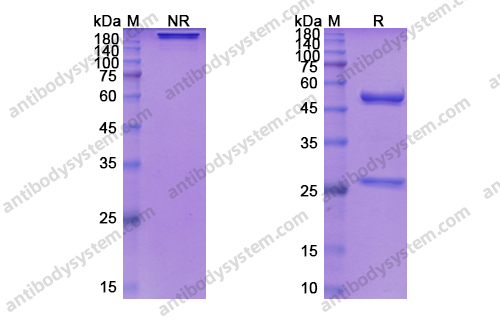
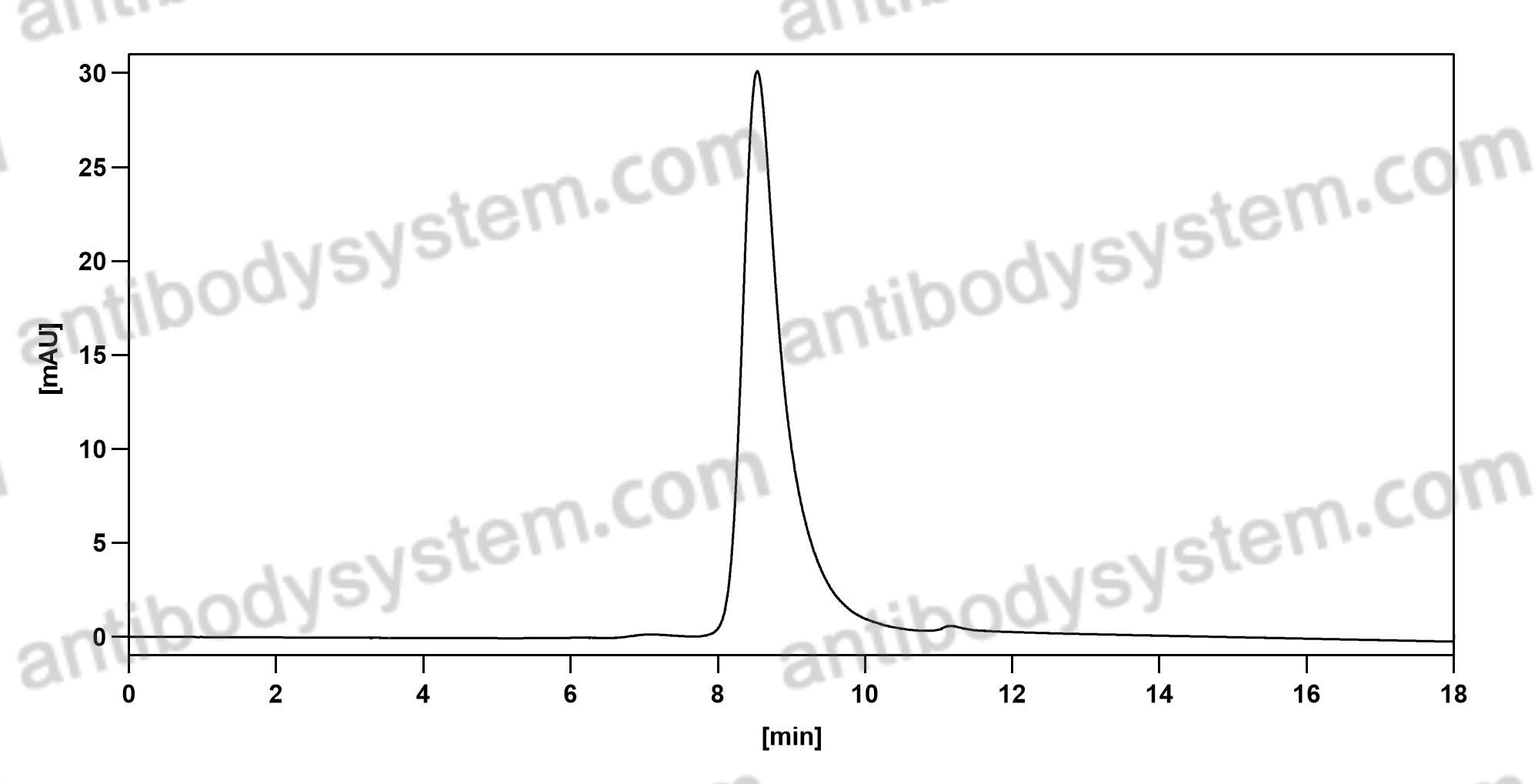
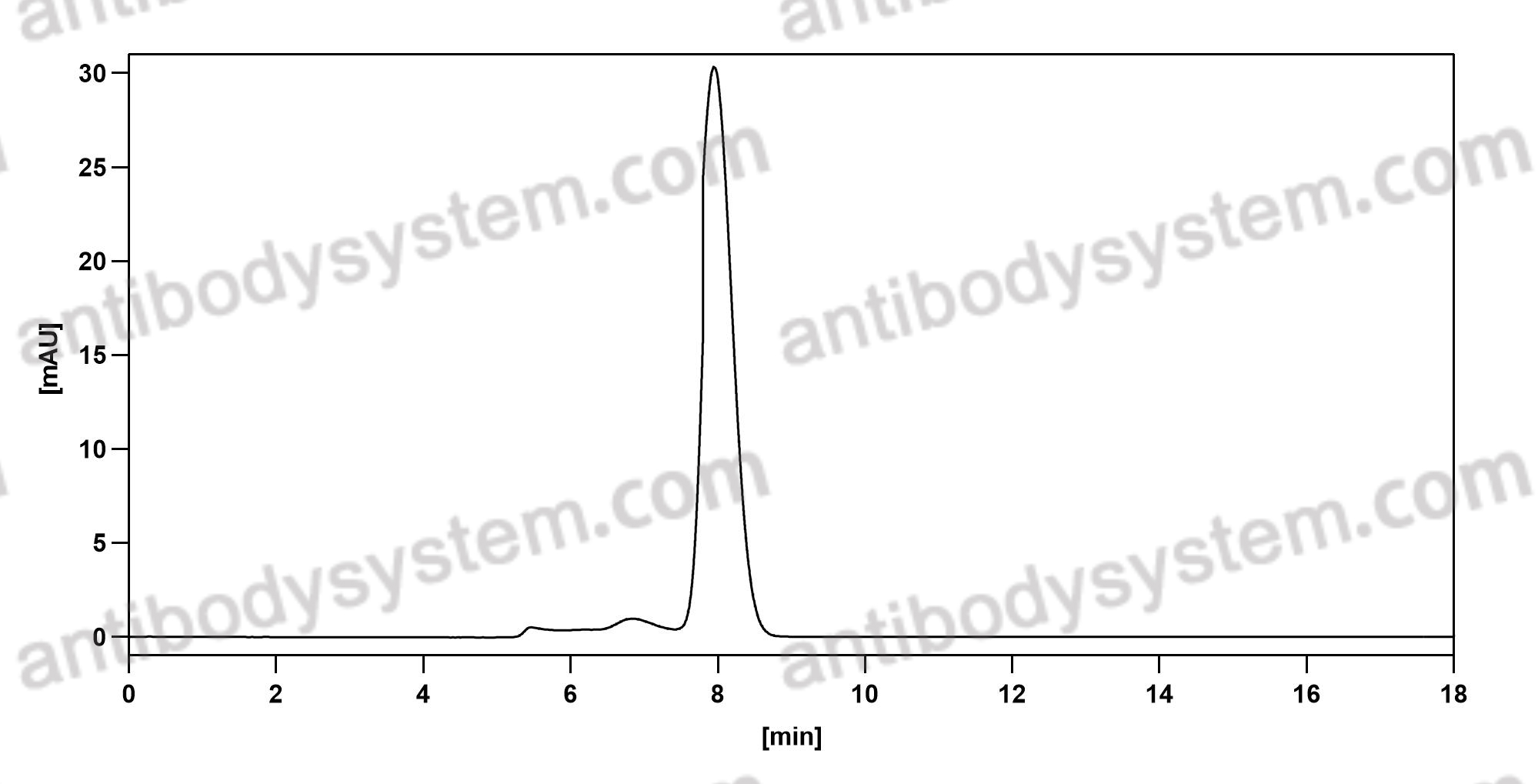
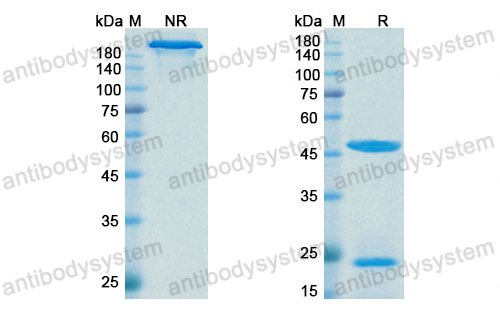
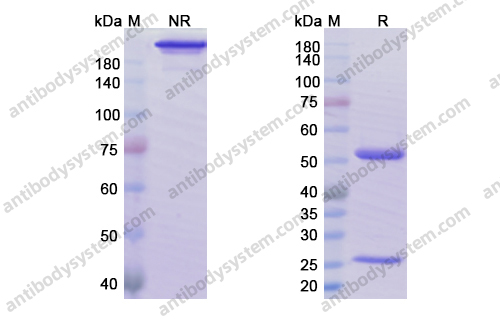
Siltuximab for multicentric Castleman's disease: a randomised, double-blind, placebo-controlled trial, PMID: 25042199
Siltuximab, PMID: 31644237
Siltuximab, PMID: 29999789
Pharmaco-Immunomodulatory Therapy in COVID-19, PMID: 32696108
Long-term safety of siltuximab in patients with idiopathic multicentric Castleman disease: a prespecified, open-label, extension analysis of two trials, PMID: 32027862
Involvement of interleukin 6 in SARS-CoV-2 infection: siltuximab as a therapeutic option against COVID-19, PMID: 32499314
Impact of siltuximab on patient-related outcomes in multicentric Castleman's disease, PMID: 29391839
Siltuximab: A Review in Idiopathic (Human Herpesvirus-8-Negative) Multicentric Castleman Disease, PMID: 26394632
Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy, PMID: 27049586
Clinical development of siltuximab, PMID: 25986720
Siltuximab and hematologic malignancies. A focus in non Hodgkin lymphoma, PMID: 28140696
Cytokine storm intervention in the early stages of COVID-19 pneumonia, PMID: 32360420
Siltuximab: first global approval, PMID: 24958337
CAR T-Cells, PMID: 32301017
Siltuximab: a new option for the management of Castleman's disease, PMID: 25685858
Siltuximab (CNTO 328): a promising option for human malignancies, PMID: 26170629
Siltuximab (Sylvant) for treatment of multicentric castleman's disease, PMID: 25555075
Siltuximab: a targeted therapy for idiopathic multicentric Castleman disease, PMID: 26634298
FDA approval: siltuximab for the treatment of patients with multicentric Castleman disease, PMID: 25601959
Population pharmacokinetics of siltuximab: impact of disease state, PMID: 31482226
Review of siltuximab in the treatment of multicentric Castleman's disease, PMID: 27904739
COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study, PMID: 32593339
Frontline Siltuximab and Rituximab in TAFRO syndrome: A case report, PMID: 32564425
The clinical, laboratory, and radiologic improvement due to siltuximab treatment in idiopathic multicentric Castleman's disease, PMID: 32088937
Treatment of Idiopathic Castleman Disease, PMID: 29157622
Interleukin-6 as a therapeutic target, PMID: 25589616
Siltuximab (Sylvant). Castleman's disease: good symptomatic efficacy in some patients, PMID: 27152394
Siltuximab in relapsed/refractory multicentric Castleman disease: Experience of the Italian NPP program, PMID: 30074630
Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Siltuximab in High-Risk Smoldering Multiple Myeloma, PMID: 30890552
Interleukin-6: A Masterplayer in the Cytokine Network, PMID: 31958792
Immune Therapy, or Antiviral Therapy, or Both for COVID-19: A Systematic Review, PMID: 33068263
Emerging agents and regimens for multiple myeloma, PMID: 33168044
Targeting interleukin-6 in inflammatory autoimmune diseases and cancers, PMID: 24076269
Targeting interleukin 6 signaling by monoclonal antibody siltuximab on cholangiocarcinoma, PMID: 33091158
International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease, PMID: 30181172
Diagnosis and Management of Castleman Disease, PMID: 31766018
Dose selection of siltuximab for multicentric Castleman's disease, PMID: 25784388
Emerging treatments in Castleman disease - a critical appraisal of siltuximab, PMID: 26869762
Novel insights and therapeutic approaches in idiopathic multicentric Castleman disease, PMID: 30487129
Role of monoclonal antibody drugs in the treatment of COVID-19, PMID: 33083387
Newly diagnosed and previously treated multicentric Castleman disease respond equally to siltuximab, PMID: 33128769
Siltuximab for multicentric Castleman disease, PMID: 25110138
Renal Toxicities of Novel Agents Used for Treatment of Multiple Myeloma, PMID: 27654928
[Effects of siltuximab on the interleukin-6/Stat3 signaling pathway in ovarian cancer], PMID: 21211286
The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer, PMID: 30372844
ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors), PMID: 29447987
Effect of siltuximab, omalizumab, infliximab, pembrolizumab and vedolizumab on selected haematological and biochemical parameters in a pig model, PMID: 32233286
Immunotherapeutic Interleukin-6 or Interleukin-6 Receptor Blockade in Cancer: Challenges and Opportunities, PMID: 28707587
Interleukin-6 inhibition in the management of non-infectious uveitis and beyond, PMID: 31523783
Siltuximab in transplant-ineligible patients with myeloma, PMID: 25121174
Management strategies for CAR-T cell therapy-related toxicities: results from a survey in Greece., PMID:40520784
[Sequential treatment with siltuximab and tocilizumab for childhood idiopathic multicentric Castleman disease: a case report]., PMID:40462437
Expert Perspective: Diagnosis and Treatment of Castleman Disease., PMID:40457814
[Siltuximab as a salvage therapy in multi-refractory adult-onset Still's disease: A case series]., PMID:40413088
Case Report: Successful use of emapalumab in adult B-cell acute lymphoblastic leukemia experiencing severe neurotoxicity and hemophagocytic lymphohistiocytosis-like features after CAR-T cell therapy., PMID:40255392
LRG1, a novel serum biomarker for iMCD disease activity., PMID:40197491
Siltuximab for the treatment of early complications after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia in children, adolescents, and young adults., PMID:40176077
Real-world data of siltuximab for Chinese patients with iMCD: combination with BCD regimen as a potential approach for severe cases., PMID:40175604
Spontaneous remission of iMCD-TAFRO: a case report., PMID:40137982
Idiopathic Multicentric Castleman's Disease Presenting with Chorioretinal Undulation: A Case Report., PMID:40101154
Inhibition of inflammation by IL-6 blockade in xenotransplantation., PMID:39999679
Insights into IL-6/JAK/STAT3 signaling in the tumor microenvironment: Implications for cancer therapy., PMID:39893129
Pericytes Promote More Vascularization than Stromal Cells via an Interleukin-6-Dependent Mechanism in Microfluidic Chips., PMID:39887579
Osteosarcoma biomarker analysis and drug targeting prediction based on pyroptosis-related genes., PMID:39833053
Multicentric Castleman's Disease presenting with bilateral panuveitis., PMID:39819875
The Value of a Pet Scan in Selecting the Best Lymph Node to Biopsy, and Confirming the Diagnosis of Idiopathic Multicentric Castleman Disease with HLH And EBV Viremia in a Previously Healthy Adult., PMID:39651386
The Enigma of Idiopathic Multicentric Castleman Disease: An Elusive Diagnosis., PMID:39650978
Cytokine testing and challenges for diagnostic and clinical monitoring use., PMID:39613111
Rapid Relapse of Idiopathic Multicentric Castleman Disease After Siltuximab Discontinuation in a Case with Complete Remission for More Than 10 Years., PMID:39501708
Siltuximab in Idiopathic Multicentric Castleman Disease: Real-World Experience., PMID:39493599
Siltuximab for chimeric antigen receptor T-cell therapy-related CRS and ICANS: a multicenter retrospective analysis., PMID:39437770
Novel use of Siltuximab in a patient with VEXAS Syndrome., PMID:39417832
Cytokine Release Syndrome Associated With Immune-Modulating Chemotherapy: Potential Mitigating Role of Intravenous Omega-3 Fatty Acid Triglycerides., PMID:39376028
Diagnostic challenges in patients with Castleman disease, a single center experience from Hungary., PMID:39252787
Unraveling the Complexities of Idiopathic Multicentric Castleman Disease and Its Multi-systemic Associations: A Case Report., PMID:39161530
IL-6 Blockade in Cytokine Storm Syndromes., PMID:39117839
Identification of critical genes and metabolic pathways in rheumatoid arthritis and osteoporosis toward drug repurposing., PMID:39079412
Successfully treated with siltuximab and prednisone in a 7-year-old girl with DOCK8-deficiency presenting as recurrent wart-like lesions: a case report., PMID:39044832
TAFRO Syndrome: Guidance for Managing Patients Presenting Thrombocytopenia, Anasarca, Fever, Reticulin Fibrosis, Renal Insufficiency, and Organomegaly., PMID:38927484
Corrigendum: Real-practice management and treatment of idiopathic multicentric Castleman disease with siltuximab: a collection of clinical experiences., PMID:38899278
Efficacy and Safety of Sintilimab Combined with Pemetrexed and Platinum Chemotherapy in Non-Small Cell Lung Cancer Patients., PMID:38870510
Idiopathic multicentric Castleman disease and connective tissue disorder successfully treated by siltuximab: a pediatric case report., PMID:38840677
Efficacy of Siltuximab and 1,927 nm Fractional Laser for the Treatment of Cutaneous Manifestations in Castleman's Disease: The Role of Dermoscopy and Reflectance Confocal Microscopy for Lesion Evaluation., PMID:38590388
Real-practice management and treatment of idiopathic multicentric Castleman disease with siltuximab: a collection of clinical experiences., PMID:38510313
A case report: Castleman disease treated by the trinity of internal and external treatment of "Fuzheng, phlegm-resolving, and detoxification method"., PMID:38306555
Text Mining and Drug Discovery Analysis: A Comprehensive Approach to Investigate Diabetes-Induced Osteoporosis., PMID:38250601
Therapy of Castleman's disease with siltuximab - case report and review of literature., PMID:38195387
Castleman's disease: one disease, multiple etiologies., PMID:39970477
Lymphadenopathy in the rheumatology practice: a pragmatic approach., PMID:38109670
Idiopathic multicentric Castleman disease: An update in diagnosis and treatment advances., PMID:38087716
Expression Analysis of Long Noncoding RNA-MALAT1 and Interleukin-6 in Inflammatory Bowel Disease Patients., PMID:38085149
Long-Term Tolerance and Efficacy of Siltuximab (Anti-IL-6) in a Young Adult with Idiopathic Multicentric Castleman Disease During COVID-19., PMID:38077710
Stepwise Treatment for TAFRO Syndrome., PMID:38029058
Castleman's disease, pathophysiology, advances in diagnosis and treatment., PMID:38016855
Siltuximab administration results in spurious IL-6 elevation in peripheral blood., PMID:37867418
A challenging diagnosis of idiopathic multicentric Castleman disease with complex systemic presentation: A case report., PMID:37854256
Molecular and Clinical Characteristics of Different Toxicity Rates in Anti-CD19 Chimeric Antigen Receptor T Cells: Real-World Experience., PMID:37686529
Treatment consistent with idiopathic multicentric Castleman disease guidelines is associated with improved outcomes., PMID:37656441