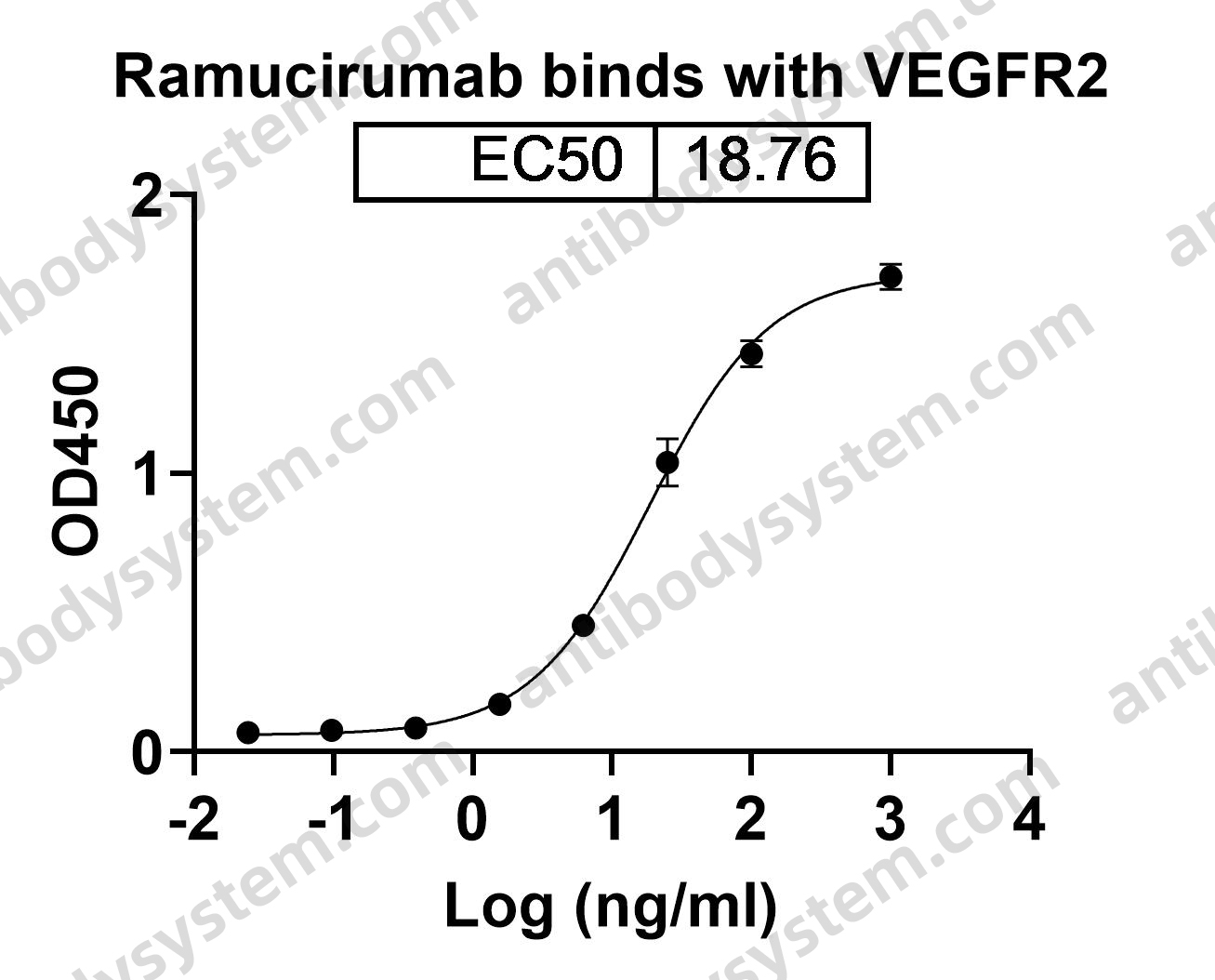
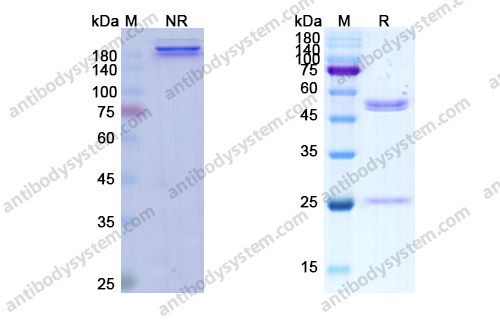
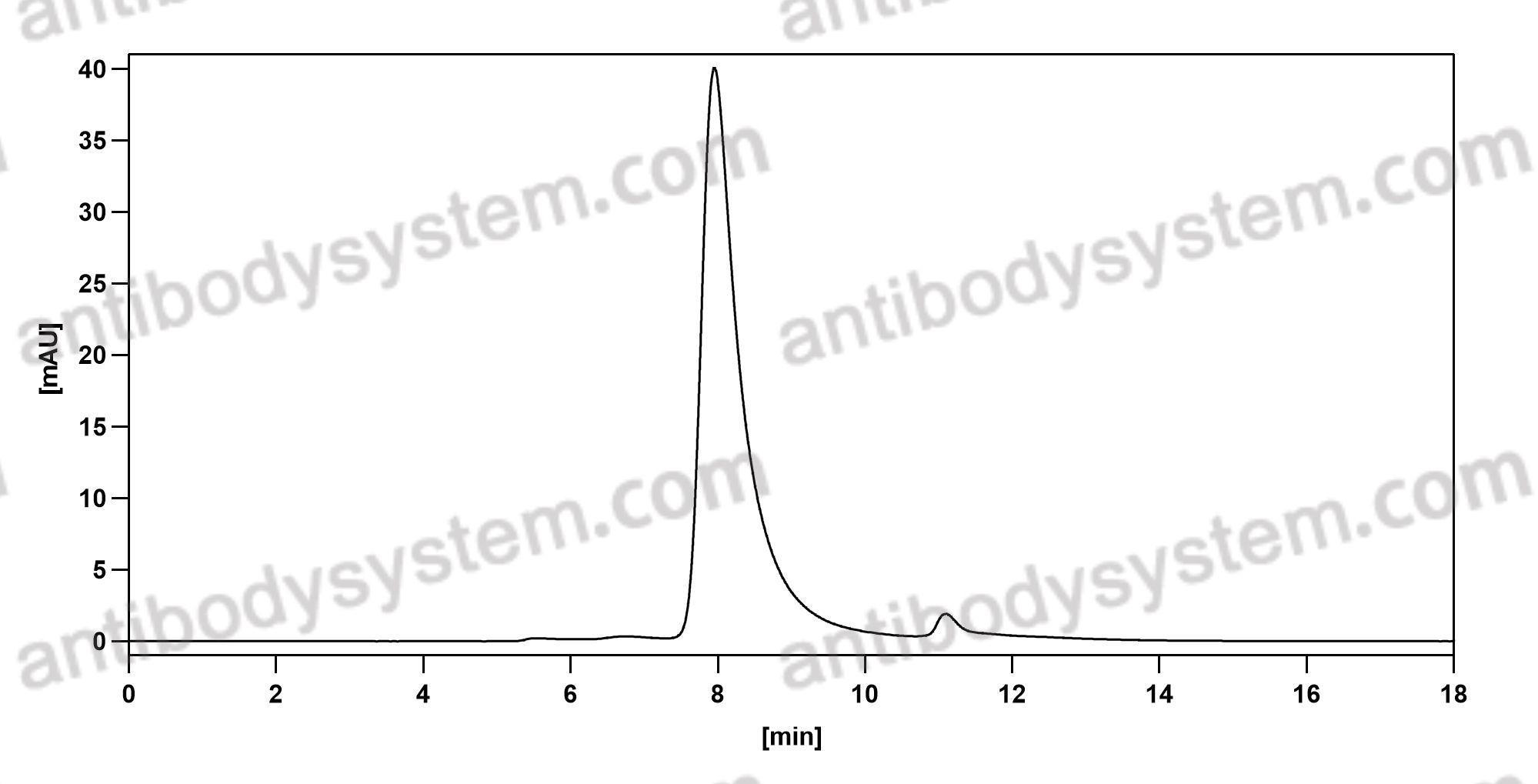
Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial, PMID: 25240821
Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial, PMID: 31591063
Ramucirumab for the treatment of gastric or gastro-esophageal junction cancer, PMID: 31452409
Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial, PMID: 24933332
Ramucirumab: A Review in Hepatocellular Carcinoma, PMID: 32034692
Ramucirumab, PMID: 27340327
Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial, PMID: 30665869
Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study, PMID: 25877855
Ramucirumab, PMID: 31643324
Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial, PMID: 24094768
Ramucirumab and its use in the treatment of hepatocellular carcinoma, PMID: 30644314
Ramucirumab, PMID: 29999742
The safety of ramucirumab for the treatment of colorectal cancer, PMID: 30073902
Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): overall survival and updated results of a randomised, double-blind, phase 3 trial, PMID: 31753727
Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial, PMID: 26095784
Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: An open-label, phase Ia/b study (JVDJ), PMID: 32827847
Ramucirumab or placebo plus erlotinib in EGFR-mutated, metastatic non-small-cell lung cancer: East Asian subset of RELAY, PMID: 32954593
Ramucirumab, A Second-Line Option For Patients With Hepatocellular Carcinoma: A Review Of The Evidence, PMID: 32547208
Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial, PMID: 30718072
Ramucirumab: the long and winding road toward being an option for mCRC treatment, PMID: 30917706
Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial, PMID: 28916371
Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF), PMID: 29853658
Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial, PMID: 31301962
Ramucirumab: Boon or bane, PMID: 27025409
[Ramucirumab - a new anticancer agent], PMID: 27690622
Targeting VEGFR2 with Ramucirumab strongly impacts effector/ activated regulatory T cells and CD8 + T cells in the tumor microenvironment, PMID: 30314524
Molecular therapies and precision medicine for hepatocellular carcinoma, PMID: 30061739
Gastric cancer, PMID: 27156933
A phase II study of nab-paclitaxel in combination with ramucirumab in patients with previously treated advanced gastric cancer, PMID: 29353164
Nephrotic syndrome associated with ramucirumab therapy: A single-center case series and literature review, PMID: 31277139
Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial, PMID: 27765757
Ramucirumab for gastric cancer, PMID: 25431958
Ramucirumab: first global approval, PMID: 24916147
Improved efficacy of taxanes and ramucirumab combination chemotherapy after exposure to anti-PD-1 therapy in advanced gastric cancer, PMID: 32719002
Ramucirumab: A Review in Advanced Gastric Cancer, PMID: 26341713
Ramucirumab as a second line therapy for advanced HCC: a significant achievement or a wasted opportunity for personalised therapy?, PMID: 30879152
The Impact of Ramucirumab Treatment on Survival and Quality of Life in Patients with Gastric Cancer, PMID: 32021422
Ramucirumab in the treatment of non-small cell lung cancer, PMID: 28395526
Ramucirumab: A vascular endothelial growth factor receptor-2 inhibitor with activity in several malignancies, PMID: 27217518
Ramucirumab: successfully targeting angiogenesis in gastric cancer, PMID: 25281695
Potential of ramucirumab in treating hepatocellular carcinoma patients with elevated baseline alpha-fetoprotein, PMID: 30464931
Osimertinib plus Ramucirumab: The Best of Both Worlds?, PMID: 33334907
A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy, PMID: 27565938
Ramucirumab Clinical Development: an Emerging Role in Gastrointestinal Tumors, PMID: 26887374
Biomarker analysis beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and second-line ramucirumab efficacy in patients with metastatic colorectal carcinoma from RAISE-a global phase III study, PMID: 30339194
Ramucirumab in elderly patients with hepatocellular carcinoma and elevated alpha-fetoprotein after sorafenib in REACH and REACH-2, PMID: 32279446
Ramucirumab for the treatment of advanced or metastatic non-small cell lung cancer, PMID: 27737562
Ramucirumab: targeting angiogenesis in the treatment of gastric cancer, PMID: 25496333
Current status of ramucirumab in gastroesophageal adenocarcinoma, PMID: 28436242
Ramucirumab for advanced gastric cancer or gastro-oesophageal junction adenocarcinoma, PMID: 26557893
Treatments and Outcomes After Platinum-Based Chemotherapy and Anti-PD-(L)1 in NSCLC., PMID:40526385
Advances in Molecular Imaging of VEGFRs: Innovations in Imaging and Therapeutics., PMID:40508182
"Attenuated" Pulmonary Tumor Thrombotic Microangiopathy on Anti-Vascular Endothelial Growth Factor Treatment: A Case Report., PMID:40475293
Association Between Baseline Neutrophil Count and Febrile Neutropenia Following Docetaxel and Ramucirumab With Prophylactic Pegfilgrastim., PMID:40468685
Final Survival Outcomes With Ramucirumab Plus Erlotinib Versus Placebo Plus Erlotinib in Patients With Untreated EGFR-Mutated Metastatic NSCLC: RELAY Japanese Subset., PMID:40458541
Trastuzumab Deruxtecan or Ramucirumab plus Paclitaxel in Gastric Cancer., PMID:40454632
EXPRESS: Advanced Therapies for Stomach Cancer., PMID:40448345
Randomized Phase III Trial of Ramucirumab Beyond Progression Plus Irinotecan in Patients With Ramucirumab-Refractory Advanced Gastric Cancer: RINDBeRG Trial., PMID:40408613
Comparison of dose rounding and drug vial optimization for reducing anticancer drug waste., PMID:40398488
Enhancing hepatocellular carcinoma treatment: synergistic cytotoxicity and mechanistic insights of Ramucirumab and 5-Azacytidine combination therapy., PMID:40397219
Real-world treatment and outcomes for EGFR WT advanced/metastatic non-squamous non-small cell lung cancer: pooled analysis from project LUMINATE-101., PMID:40338221
Therapeutic management of patients with advanced thymic malignancies: A review for clinicians., PMID:40334289
Maintenance Capecitabine Plus Ramucirumab After First-Line Chemotherapy in Patients With Advanced Esophagogastric Adenocarcinoma: Results From the Randomized PLATFORM Study., PMID:40330143
Unravelling Paclitaxel Resistance in Gastric Cancer: The Role of Small Extracellular Vesicles in Epithelial Mesenchymal Transition and Extracellular Matrix Remodelling., PMID:40282535
Thromboembolic and bleeding events associated with angiogenesis inhibitors in cancer patients., PMID:40246589
A disproportionality analysis of interstitial lung disease associated with drug therapy in spontaneous adverse event reports., PMID:40232264
Toxicity Profile and Efficacy of Docetaxel Following Paclitaxel- or Pemetrexed-Platinum Chemotherapy Alone or in Combination With Immune Checkpoint Inhibitors in NSCLC Patients: A Single Institution Retrospective Analysis., PMID:40220012
[A Case of Long-Term Prognosis of Advanced Rectosigmoid Cancer with Multiple Metastases Treated with Multidisciplinary Therapy]., PMID:40189763
S2303: phase II/III trial of paclitaxel + ramucirumab ± nivolumab in gastric and esophageal adenocarcinoma (PARAMUNE)., PMID:40155326
Ramucirumab and paclitaxel in second or greater lines of therapy in patients with HER2-positive gastroesophageal cancer: a single center study., PMID:40152313
Pairwise analysis of plasma cell-free DNA before and after palliative second-line paclitaxel plus ramucirumab treatment in patients with metastatic gastric cancer., PMID:40148708
Comparison of the prognostic effect of taxane regimens combined with ramucirumab before nivolumab for advanced gastric cancer., PMID:40095335
Successful Desensitization to Ramucirumab in Signet-Cell Gastric Adenocarcinoma., PMID:40079904
Nab-paclitaxel combined with cadonilimab (AK104) as second-line treatment for advanced gastric cancer: protocol for a phase II prospective, multicenter, single-arm clinical trial., PMID:40070819
Correction: Clinical Significance of Prior Ramucirumab Use on the Effectiveness of Nivolumab as the Third-Line Regimen in Gastric Cancer: A Multicenter Retrospective Study., PMID:40067648
Complete Remission with Nivolumab Monotherapy of Advanced Gastric Neuroendocrine Carcinoma., PMID:40024686
Drug-Induced Chylothorax During Chemotherapy With Ramucirumab and Paclitaxel for Advanced Gastric Cancer., PMID:39991332
In vitro Stability Study of a Panel of Commercial Antibodies at Physiological pH and Temperature as a Guide to Screen Biologic Candidate Molecules for the Potential Risk of In vivo Asparagine Deamidation and Activity Loss., PMID:39979532
[A Case of Multiple Brain Metastasis after Resection of Gastric Cancer with Adrenal Metastasis]., PMID:39948918
Monoclonal Antibodies and Small-Molecule Inhibitors Associated Osteonecrosis of Jaw: A Retrospective Pharmacovigilance Study., PMID:39922223
Phase II trial of nab-paclitaxel plus ramucirumab in combination with nivolumab for unresectable advanced or recurrent gastric cancer after progression on first-line treatment including fluoropyrimidine, platinum, and anti-PD-1/PD-L1 antibody (PADDLE)., PMID:39905373
A Case of Purpuric Papulopustular Eruption on the Extremities Developed During Erlotinib and Ramucirumab Combination Treatment, Resulting in Complete Regression Without Oral Prednisone or Discontinuing Chemotherapy., PMID:39881923
Efficacy of ramucirumab combined with erlotinib or osimertinib in untreated EGFR-mutated NSCLC patients with asymptomatic brain metastases: insights from molecular biomarkers in the RELAY-brain trial., PMID:39847172
A phase II study of ramucirumab and somatostatin analog therapy in patients with advanced neuroendocrine tumors., PMID:39834129
A non-interventional biomarker study in patients with adenocarcinoma of the lung treated with nintedanib plus docetaxel following progression on chemotherapy and/or immunotherapy: LUME-BioNIS., PMID:39830745
Development of treatment strategies for advanced HER2-positive gastric cancer: Insights from clinical trials., PMID:39805409
The Efficacy of FOLFIRI Plus Ramucirumab in Recurrent Colorectal Cancer Refractory to Adjuvant Chemotherapy with Oxaliplatin/Fluoropyrimidine-Including Biomarker Analyses., PMID:39796720
Immune Microenvironment and the Effect of Vascular Endothelial Growth Factor Inhibition in Hepatocellular Carcinoma., PMID:39769351
Circulating miR-23b-3p, miR-30e-3p, and miR-205-5p as Novel Predictive Biomarkers for Ramucirumab-Paclitaxel Therapy Outcomes in Advanced Gastric Cancer., PMID:39769259
Addressing the unmet need in NSCLC progression with advances in second-line therapeutics., PMID:39759220
[A Case of Pathological Complete Response after Nivolumab Therapy in Unresectable Advanced Gastric Cancer]., PMID:39721791
Alternating Administration of Osimertinib for Leptomeningitis and Docetaxel Plus Ramucirumab for Lung Adenocarcinoma Resistant to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: A Case Report., PMID:39721695
What is the optimal first-line regimen for advanced non-small cell lung cancer patients with epidermal growth factor receptor mutation: a systematic review and network meta-analysis., PMID:39695621
A real-world disproportionality analysis of FDA Adverse Event Reporting System (FAERS) events for ramucirumab., PMID:39656165
Advances in systemic therapy leading to conversion surgery for advanced hepatocellular carcinoma., PMID:39647858
Impact of ABCB1 single-nucleotide variants on early, extremely severe neutropenia induced by paclitaxel/nanoparticle albumin-bound paclitaxel in patients with gastric cancer., PMID:39622587
RELAY: Final Overall Survival for Erlotinib Plus Ramucirumab or Placebo in Untreated, EGFR-Mutated Metastatic NSCLC., PMID:39622410
S1701, A Randomized Phase 2 Trial of Carboplatin-Paclitaxel With and Without Ramucirumab in Patients With Locally Advanced, Recurrent, or Metastatic Thymic Carcinoma., PMID:39619274
A case of complete remission by cabozantinib as an end-line treatment for advanced hepatocellular carcinoma., PMID:39616585
Clinicopathological analysis of claudin 18.2 focusing on intratumoral heterogeneity and survival in patients with metastatic or unresectable gastric cancer., PMID:39615405