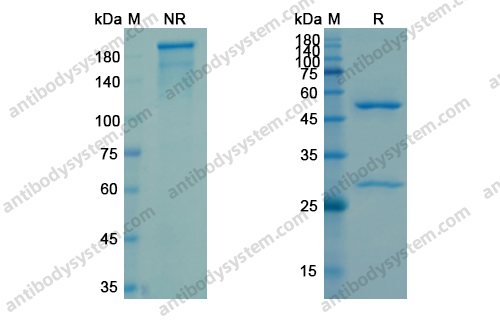
Raxibacumab, PMID: 20068396
Raxibacumab: a panacea for anthrax disease?, PMID: 32333846
Raxibacumab for anthrax, PMID: 23545582
Raxibacumab: potential role in the treatment of inhalational anthrax, PMID: 24812521
Raxibacumab, PMID: 33886175
Effect of raxibacumab on immunogenicity of Anthrax Vaccine Adsorbed: a phase 4, open-label, parallel-group, randomised non-inferiority study, PMID: 32333847
Raxibacumab for the treatment of inhalational anthrax, PMID: 19587338
Raxibacumab for inhalational anthrax: an effective specific therapeutic approach?, PMID: 20450444
Raxibacumab augments hemodynamic support and improves outcomes during shock with B. anthracis edema toxin alone or together with lethal toxin in canines, PMID: 26097803
Added benefit of raxibacumab to antibiotic treatment of inhalational anthrax, PMID: 25487792
Approval of Raxibacumab for the Treatment of Inhalation Anthrax Under the US Food and Drug Administration "Animal Rule", PMID: 26648915
Population pharmacokinetics of raxibacumab in healthy adult subjects, PMID: 33973655
Pharmacology and Anti-infective Role of Raxibacumab: A Novel Monoclonal Antibody for the Treatment of Anthrax, PMID: 28358441
Teriflunomide, PMID: 24421467
Extrapolation and dosing recommendations for raxibacumab in children from birth to age <18 years, PMID: 33974281
Anthrax Antitoxins, PMID: 31644058
Bacillus anthracis protective antigen kinetics in inhalation spore-challenged untreated or levofloxacin/ raxibacumab-treated New Zealand white rabbits, PMID: 23344456
Estimation of Time Period for Effective Human Inhalational Anthrax Treatment Including Antitoxin Therapy, PMID: 28856066
Obiltoxaximab (Anthim) for inhalation anthrax, PMID: 30383733
Bacillus anthracis infections--new possibilities of treatment, PMID: 26094508
Designing inhibitors of anthrax toxin, PMID: 24447197
Pharmaceutical approval update, PMID: 23599674
Development trends for human monoclonal antibody therapeutics, PMID: 20811384
A systematic review and meta-analysis of preclinical trials testing anti-toxin therapies for B. anthracis infection: A need for more robust study designs and results, PMID: 28797061
Monoclonal Antibodies Against Infectious Microbes: So Long and Too Little!, PMID: 32164518
Antitoxin Treatment of Inhalation Anthrax: A Systematic Review, PMID: 26690378
A Review of the Efficacy of FDA-Approved B. anthracis Anti-Toxin Agents When Combined with Antibiotic or Hemodynamic Support in Infection- or Toxin-Challenged Preclinical Models, PMID: 33450877
Alternative pre-approved and novel therapies for the treatment of anthrax, PMID: 27809794
Bacillus anthracis Edema Toxin Increases Fractional Free Water and Sodium Reabsorption in an Isolated Perfused Rat Kidney Model, PMID: 28438974
Passive Immunotherapy Protects against Enteric Invasion and Lethal Sepsis in a Murine Model of Gastrointestinal Anthrax, PMID: 26426050
Pediatric anthrax clinical management: executive summary, PMID: 24777221
Protecting against future shock--inhalational anthrax, PMID: 19587345
Animal rule for drug approval creates a jungle of confusion, PMID: 23389593
Special considerations for prophylaxis for and treatment of anthrax in pregnant and postpartum women, PMID: 24457117
Centers for disease control and prevention expert panel meetings on prevention and treatment of anthrax in adults, PMID: 24447897
New drugs 2014, part 2, PMID: 24905857
United States' regulatory approved pharmacotherapies for nuclear reactor explosions and anthrax-associated bioterrorism., PMID:37594915
Survival of Patient With Hemorrhagic Meningitis Associated With Inhalation Anthrax., PMID:36251557
Pre- and Postlicensure Animal Efficacy Studies Comparing Anthrax Antitoxins., PMID:36251555
A Novel Toll-Like Receptor 2 Agonist Protects Mice in a Prophylactic Treatment Model Against Challenge With Bacillus anthracis., PMID:35369443
Exotoxin-Targeted Drug Modalities as Antibiotic Alternatives., PMID:35099182
Monoclonal Antibodies for Protozoan Infections: A Future Reality or a Utopic Idea?, PMID:34712683
Extrapolation and dosing recommendations for raxibacumab in children from birth to age <18 years., PMID:33974281
Population pharmacokinetics of raxibacumab in healthy adult subjects., PMID:33973655
A Review of the Efficacy of FDA-Approved B. anthracis Anti-Toxin Agents When Combined with Antibiotic or Hemodynamic Support in Infection- or Toxin-Challenged Preclinical Models., PMID:33450877
Effect of raxibacumab on immunogenicity of Anthrax Vaccine Adsorbed: a phase 4, open-label, parallel-group, randomised non-inferiority study., PMID:32333847
Raxibacumab: a panacea for anthrax disease?, PMID:32333846
Monoclonal Antibodies Against Infectious Microbes: So Long and Too Little!, PMID:32164518
Obiltoxaximab (Anthim) for inhalation anthrax., PMID:30383733
Estimation of Time Period for Effective Human Inhalational Anthrax Treatment Including Antitoxin Therapy., PMID:28856066
A systematic review and meta-analysis of preclinical trials testing anti-toxin therapies for B. anthracis infection: A need for more robust study designs and results., PMID:28797061
Bacillus anthracis Edema Toxin Increases Fractional Free Water and Sodium Reabsorption in an Isolated Perfused Rat Kidney Model., PMID:28438974
Alternative pre-approved and novel therapies for the treatment of anthrax., PMID:27809794
Pharmacology and Anti-infective Role of Raxibacumab: A Novel Monoclonal Antibody for the Treatment of Anthrax., PMID:28358441
Antitoxin Treatment of Inhalation Anthrax: A Systematic Review., PMID:26690378
Approval of Raxibacumab for the Treatment of Inhalation Anthrax Under the US Food and Drug Administration "Animal Rule"., PMID:26648915
Passive Immunotherapy Protects against Enteric Invasion and Lethal Sepsis in a Murine Model of Gastrointestinal Anthrax., PMID:26426050
Raxibacumab augments hemodynamic support and improves outcomes during shock with B. anthracis edema toxin alone or together with lethal toxin in canines., PMID:26097803
Bacillus anthracis infections--new possibilities of treatment., PMID:26094508
Added benefit of raxibacumab to antibiotic treatment of inhalational anthrax., PMID:25487792
New drugs 2014, part 2., PMID:24905857
Raxibacumab: potential role in the treatment of inhalational anthrax., PMID:24812521
Pediatric anthrax clinical management: executive summary., PMID:24777221
Special considerations for prophylaxis for and treatment of anthrax in pregnant and postpartum women., PMID:24457117
Centers for disease control and prevention expert panel meetings on prevention and treatment of anthrax in adults., PMID:24447897
Designing inhibitors of anthrax toxin., PMID:24447197
Teriflunomide., PMID:24421467
Pharmaceutical approval update., PMID:23599674
Raxibacumab for anthrax., PMID:23545582
Animal rule for drug approval creates a jungle of confusion., PMID:23389593
Bacillus anthracis protective antigen kinetics in inhalation spore-challenged untreated or levofloxacin/ raxibacumab-treated New Zealand white rabbits., PMID:23344456
Development trends for human monoclonal antibody therapeutics., PMID:20811384
Raxibacumab for inhalational anthrax: an effective specific therapeutic approach?, PMID:20450444
Raxibacumab., PMID:20068396
Protecting against future shock--inhalational anthrax., PMID:19587345
Raxibacumab for the treatment of inhalational anthrax., PMID:19587338