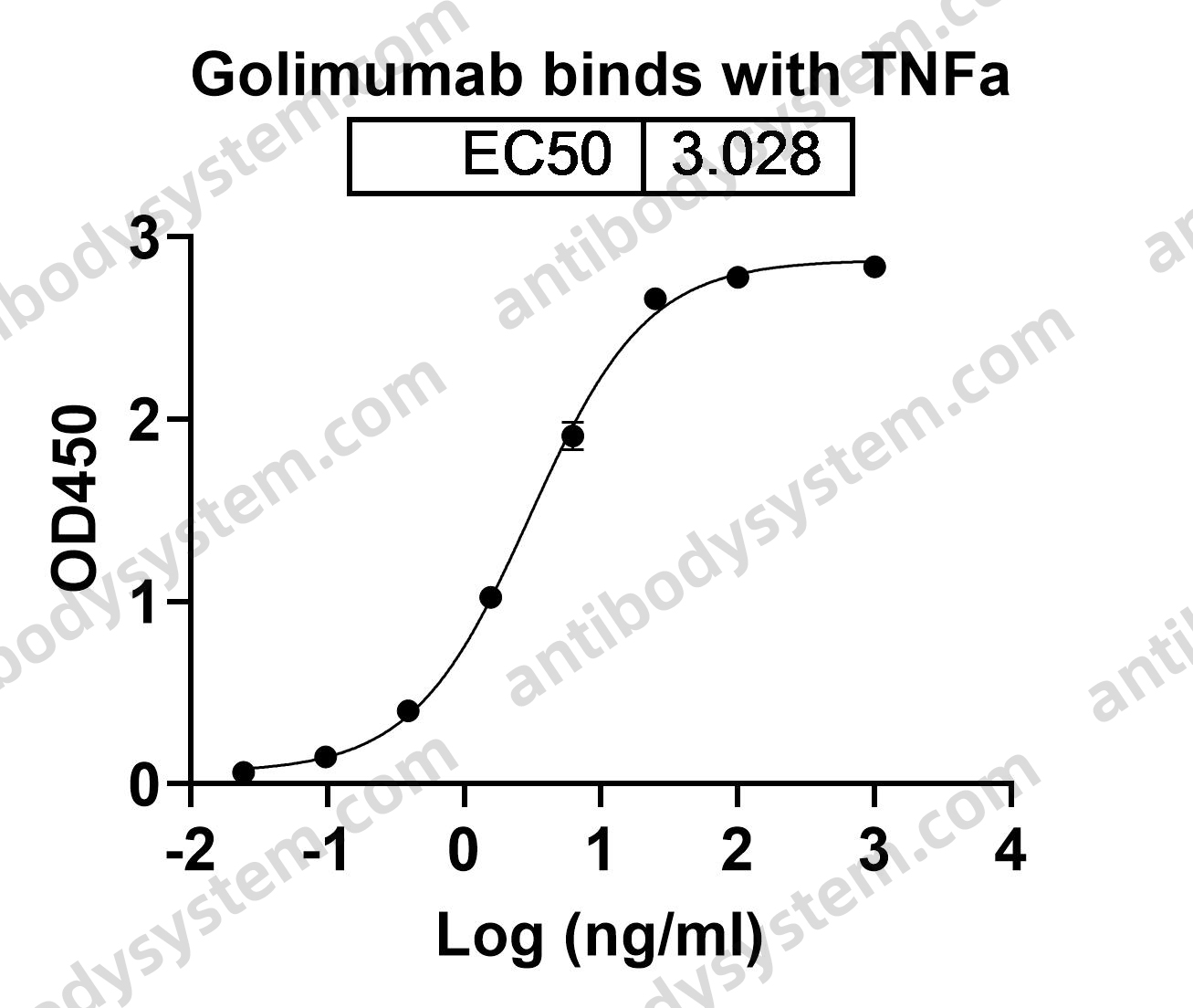
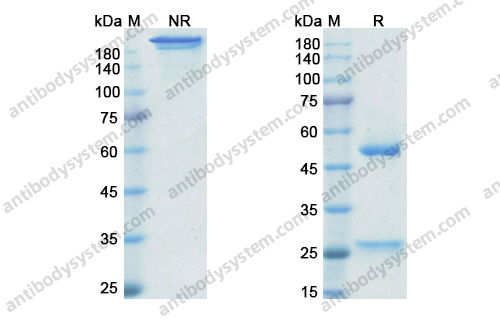
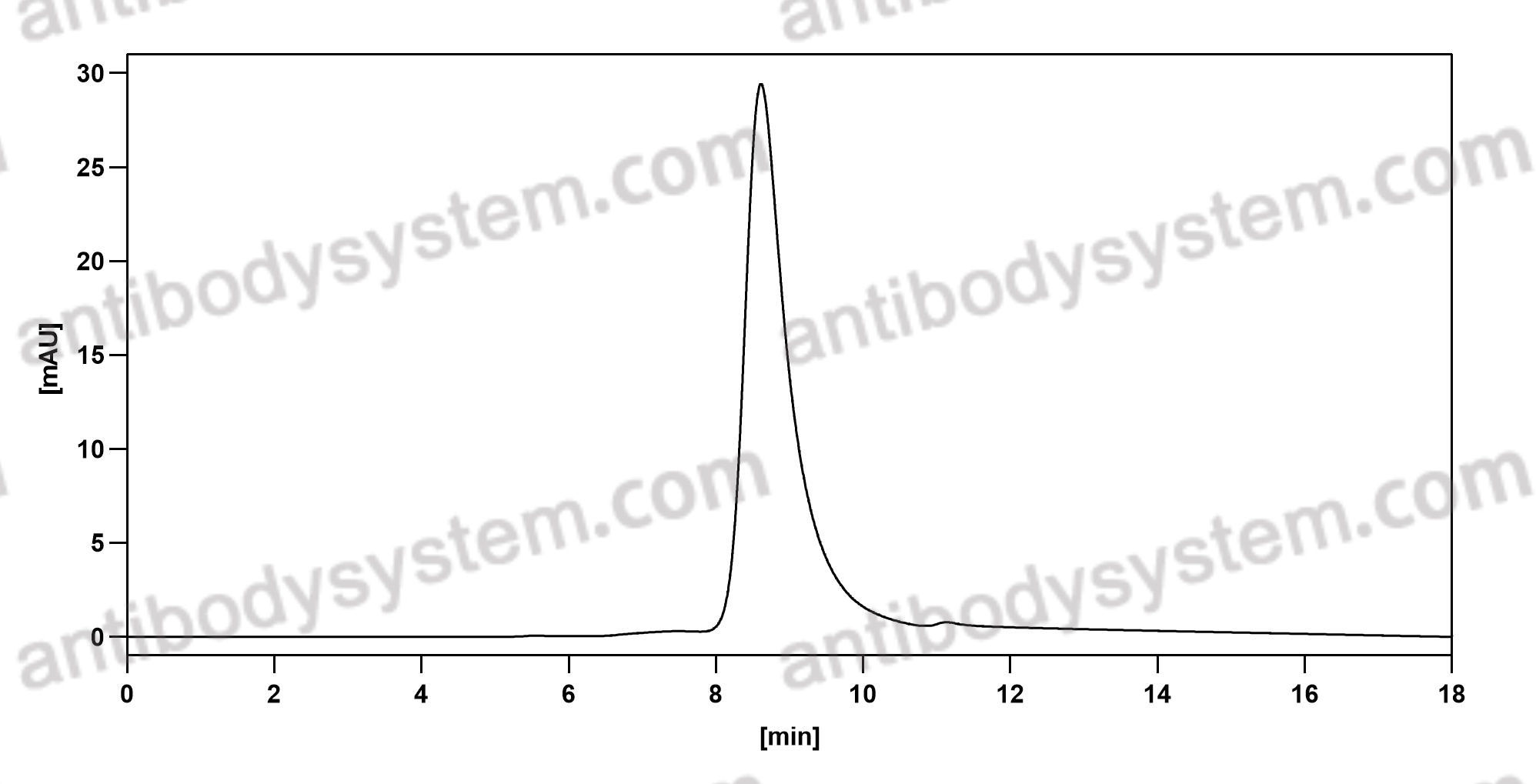
Golimumab and Beta-Cell Function in Youth with New-Onset Type 1 Diabetes, PMID: 33207093
[Golimumab], PMID: 23961671
Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis, PMID: 23735746
Golimumab, PMID: 20065639
Golimumab in the treatment of psoriatic arthritis, PMID: 30221556
Golimumab (anti-TNF monoclonal antibody): where we stand today, PMID: 33369527
Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis, PMID: 23770005
Golimumab in inflammatory bowel diseases: present and future scenarios, PMID: 30206776
Golimumab: A Review in Inflammatory Arthritis, PMID: 28530020
Golimumab for the treatment of ulcerative colitis, PMID: 28472597
Golimumab for the treatment of axial spondyloarthritis, PMID: 27817204
Golimumab for rheumatoid arthritis, PMID: 20091667
Golimumab for moderately to severely active ulcerative colitis, PMID: 27498886
Patients with ankylosing spondylitis treatment by golimumab: a systematic review and meta-analysis, PMID: 32447529
Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study, PMID: 19066176
A review on golimumab in the treatment of psoriatic arthritis, PMID: 28838285
[Golimumab Therapy in Ulcerative Colitis], PMID: 26907481
Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial, PMID: 19560810
Golimumab, PMID: 19721444
Golimumab for treatment of axial spondyloarthritis, PMID: 26798943
Efficacy and safety of certolizumab pegol and golimumab in the treatment of non-infectious uveitis, PMID: 30943133
Effectiveness of golimumab in ulcerative colitis: A review of the real world evidence, PMID: 30555013
Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial, PMID: 18975305
Golimumab for the treatment of axial spondyloarthritis, PMID: 26523483
GO-DACT: a phase 3b randomised, double-blind, placebo-controlled trial of GOlimumab plus methotrexate (MTX) versus placebo plus MTX in improving DACTylitis in MTX-naive patients with psoriatic arthritis, PMID: 32193187
Golimumab in adolescents with Crohn's disease refractory to previous tumour necrosis factor antibody, PMID: 32781480
Golimumab, the newest TNF-α blocker, comes of age, PMID: 25602858
Golimumab for moderate to severe ulcerative colitis, PMID: 28276288
Golimumab for the treatment of psoriatic arthritis, PMID: 21609657
Golimumab and immunogenicity? 2010 and beyond, PMID: 21612149
Golimumab for rheumatoid arthritis: a systematic review, PMID: 20436075
Intravenous golimumab in rheumatoid arthritis, PMID: 24831189
Golimumab: clinical update on its use for ulcerative colitis, PMID: 25876561
Therapeutic Drug Monitoring of Golimumab in the Treatment of Ulcerative Colitis, PMID: 28374338
Golimumab and malignancies: true or false association?, PMID: 20373059
Golimumab in real-world practice in patients with ulcerative colitis: Twelve-month results, PMID: 32550760
Adverse effects of golimumab in the treatment of rheumatologic diseases, PMID: 23984970
An appraisal of golimumab in the treatment of severe, active nonradiographic axial spondyloarthritis, PMID: 27468228
Evaluation of golimumab for the treatment of patients with active rheumatoid arthritis, PMID: 26811250
Letter: golimumab efficacy in patients with Crohn's disease and concomitant severe arthritis, PMID: 32445531
Golimumab for the treatment of ankylosing spondylitis: a NICE single technology appraisal, PMID: 23580355
Golimumab improves patient-reported outcomes in daily practice of inflammatory rheumatic diseases in Germany, PMID: 32722921
Whither Type 1 Diabetes?, PMID: 33207099
Golimumab therapy of rheumatoid arthritis: an overview, PMID: 20618765
Golimumab: a tumor necrosis factor alpha inhibitor for the treatment of rheumatoid arthritis, PMID: 20118145
Golimumab: in the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, PMID: 19489653
[Clinical analysis of golimumab in the treatment of severe/refractory cardiovascular involvement in Behcet syndrome], PMID: 33331313
Golimumab effectiveness in biologic inadequate responding patients with rheumatoid arthritis, psoriatic arthritis and spondyloarthritis in real-life from the Italian registry GISEA, PMID: 32755721
Why golimumab in the treatment of psoriatic arthritis, ankylosing spondylitis and rheumatoid arthritis?, PMID: 25829189
Golimumab in radiographic and nonradiographic axial spondyloarthritis: a review of clinical trials, PMID: 27445459
Influence of Pharmacokinetic-Pharmacodynamic Failure Mechanisms on Outcomes in Biologic Therapy Sequencing in Inflammatory Bowel Disease: A Retrospective Cohort Study., PMID:40531271
IGF2BP2 regulates inflammation in ulcerative colitis through N6-methyladenosine-dependent modulation of CBR1., PMID:40526983
Efficacy of Golimumab in Early Axial Spondyloarthritis in Relation to Gut Inflammation (GO-GUT), an Early Remission Induction Study., PMID:40524500
Treatment sequences, outcomes, healthcare utilization, and costs in patients with inflammatory bowel diseases requiring advanced treatment-real world comparative effectiveness from German claims data., PMID:40481399
Pharmacogenomics of TNF inhibitors., PMID:40469293
Experience of High Tibial Osteotomy for Patients with Rheumatoid Arthritis Treated with Recent Medication: A Case Series., PMID:40429328
The Relationship Between TNF-α Inhibitor Potency and HBV Reactivation in Patients With Rheumatic Disorders., PMID:40418092
Systematic review and meta-analysis of the efficacy of biologic and targeted synthetic therapies in sarcoidosis., PMID:40393718
Golimumab retention in patients with psoriatic arthritis and axial spondyloarthritis: evidence from up to a decade of follow-up., PMID:40380936
Drug-associated infections and infestations in older adults with tumor necrosis factor-alpha inhibitors: a real-world retrospective and pharmacovigilance study., PMID:40371340
Real-World Insights From Türkiye: Biologic DMARDs Usage in Spondyloarthritis Patients With Chronic Kidney Disease., PMID:40358366
Aggregate Distributional Cost-Effectiveness Analysis of Biologics for the Treatment of Ankylosing Spondylitis in Chile., PMID:40329067
Prevalence of opportunistic infections in Syrian inflammatory bowel disease patients on biologic therapy: a multi-center retrospective cross-sectional study., PMID:40320559
Unveiling the differences: infection disorders associated with tumor necrosis factor α inhibitors in pediatric patients-a pharmacovigilance study (2004-2023)., PMID:40317305
Diffusion dimensionality modeling of subcutaneous/intramuscular absorption of antibodies and long-acting injectables., PMID:40263185
Racial Disparities in Utilization of Medications and Disease Outcomes in Inflammatory Bowel Disease Patients., PMID:40260307
Recommendations for the Use of Disease-Modifying Antirheumatic Drugs in Pregnancy and Reproductive Health for Patients With Rheumatic Disease: A Scoping Review., PMID:40256995
Characterising infusion/injection-related reactions in patients with rheumatoid arthritis treated with biologic agents., PMID:40249052
Relapsing-remitting multiple sclerosis as a potential consequence of thalidomide treatment: A case report., PMID:40245782
Immunotherapy as a treatment for type 1 diabetes mellitus in children and young adults: A comprehensive systematic review and meta-analysis., PMID:40215252
Dual biological treatments in immune-mediated disorders: a single center experience., PMID:40200173
Rapid, Single-Step Monitoring of Monoclonal Antibody Bioavailability by Using a TNF-α-Based Multiepitope DNA Nanoswitch., PMID:40198205
Biosimilars versus biological therapy in inflammatory bowel disease: challenges and targeting strategies using drug delivery systems., PMID:40186719
Infection toxicity assessment of tumor necrosis factor α inhibitors in the treatment of IBD: a real-world study based on the US food and drug administration adverse events reporting system (FAERS)., PMID:40156444
Treatment of uveitis in Blau syndrome: A systematic review and meta-analysis., PMID:40147219
Abatacept, Golimumab, and Sarilumab as Selected Bio-Originator Disease-Modifying Antirheumatic Drugs with Diverse Mechanisms of Action in Their Current Use in Treatment., PMID:40142915
Biological Disease-Modifying Antirheumatic Drugs Decrease Uric Acid Levels in the Sera of Patients with Psoriatic Arthritis., PMID:40136396
Guselkumab versus golimumab in patients with active psoriatic arthritis and inadequate response to an initial tumor necrosis factor inhibitor: study protocol for EVOLUTION, a pragmatic, phase 3b, open-label, randomized, controlled effectiveness trial., PMID:40102973
Effect of TNF-α blockers on reducing the risk of dementia in rheumatoid arthritis: a nationwide cohort study., PMID:40095620
Anti-Rheumatic potential of biological DMARDS and protagonistic role of bio-markers in early detection and management of rheumatoid arthritis., PMID:40091354
Golimumab-induced lichen planus pigmentosus in a patient with ulcerative colitis., PMID:40087062
Risk of hematologic malignancies in psoriasis and rheumatoid arthritis patients using long term TNF-α inhibitors: a retrospective nationwide study., PMID:40055388
Dose Escalation Patterns and Associated Costs of Advanced Therapies for Ulcerative Colitis in France and the United Kingdom: A Retrospective Database Analysis., PMID:40046402
Conducting a real-world study of Tumor Necrosis factor-alpha inhibitors-induced Systemic Lupus Erythematosus based on the FAERS database., PMID:40000785
In vitro Stability Study of a Panel of Commercial Antibodies at Physiological pH and Temperature as a Guide to Screen Biologic Candidate Molecules for the Potential Risk of In vivo Asparagine Deamidation and Activity Loss., PMID:39979532
Comparative Effectiveness and Safety of the JAK Inhibitors and Biologic Disease-Modifying Antirheumatic Drugs in Treating Children With Nonsystemic Juvenile Idiopathic Arthritis: A Bayesian Meta-Analysis of Randomized Controlled Trials., PMID:39964338
Cost-Effectiveness Analysis of Upadacitinib in Patients With Moderately to Severely Active Ulcerative Colitis in Greece., PMID:39954537
An update on the safety of biologic therapies for the treatment of polyarticular juvenile idiopathic arthritis., PMID:39946290
Tumor Necrosis Factor-Alpha Inhibitor Use and Malignancy Risk: A Systematic Review and Patient Level Meta-Analysis., PMID:39941759
A Longitudinal Post-authorization Safety Study of Golimumab in Treatment of Ulcerative Colitis: A Cohort Study in Denmark and Sweden, 2013-2021., PMID:39913070
Incidence rates of tuberculosis and inflammatory bowel disease in patients with ankylosing spondylitis treated with biologics in Korea., PMID:39854270
Successful Treatment of Refractory Ulcerative Colitis With 5-Aminosalicylic Acid Intolerance and Biologic Therapy Resistance Using Combined Granulocyte and Monocyte Adsorptive Apheresis., PMID:39834662
Paradoxical Inflammatory Bowel Disease Induced by Golimumab in a Patient With Ankylosing Spondylitis: A Case Report and Systematic Review., PMID:39807347
Construct validity and responsiveness of ASAS Health Index assessed in two longitudinal studies of tumour necrosis factor alpha inhibitor initiation and dose reduction in patients with axial spondyloarthritis., PMID:39794273
Real-World Persistence and Effectiveness of Upadacitinib versus Other Janus Kinase Inhibitors and Tumor Necrosis Factor Inhibitors in Australian Patients with Rheumatoid Arthritis., PMID:39757285
Treatment Persistence Among Anti-Tumor Necrosis Factor-experienced Patients With Ulcerative Colitis Switching to a Biologic With a Different Mode of Action or Cycling to Another Anti-Tumor Necrosis Factor Agent., PMID:39743427
Uncovering novel therapeutic clues for hypercoagulable active ulcerative colitis: novel findings from old data., PMID:39735422
Effectiveness and Treatment Persistence of Vedolizumab Compared to Anti-Tumour Necrosis Factor-α in Patients With Crohn's Disease: A Systematic Literature Review and Meta-Analysis., PMID:39707930
Persistence of advanced therapies in patients with inflammatory bowel disease: retrospective cohort study using a large healthcare claims database in Japan., PMID:39701922
Pulmonary toxicity assessment of tumor necrosis factor α inhibitors in the treatment of IBD: a real world study based on US food and drug administration adverse events reporting system (FAERS)., PMID:39695351