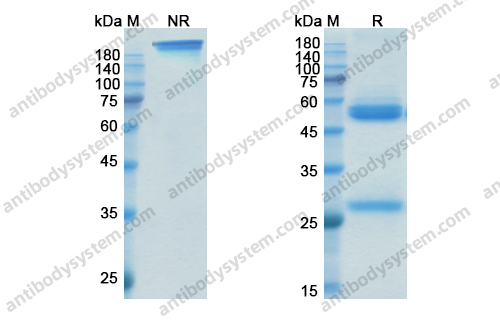
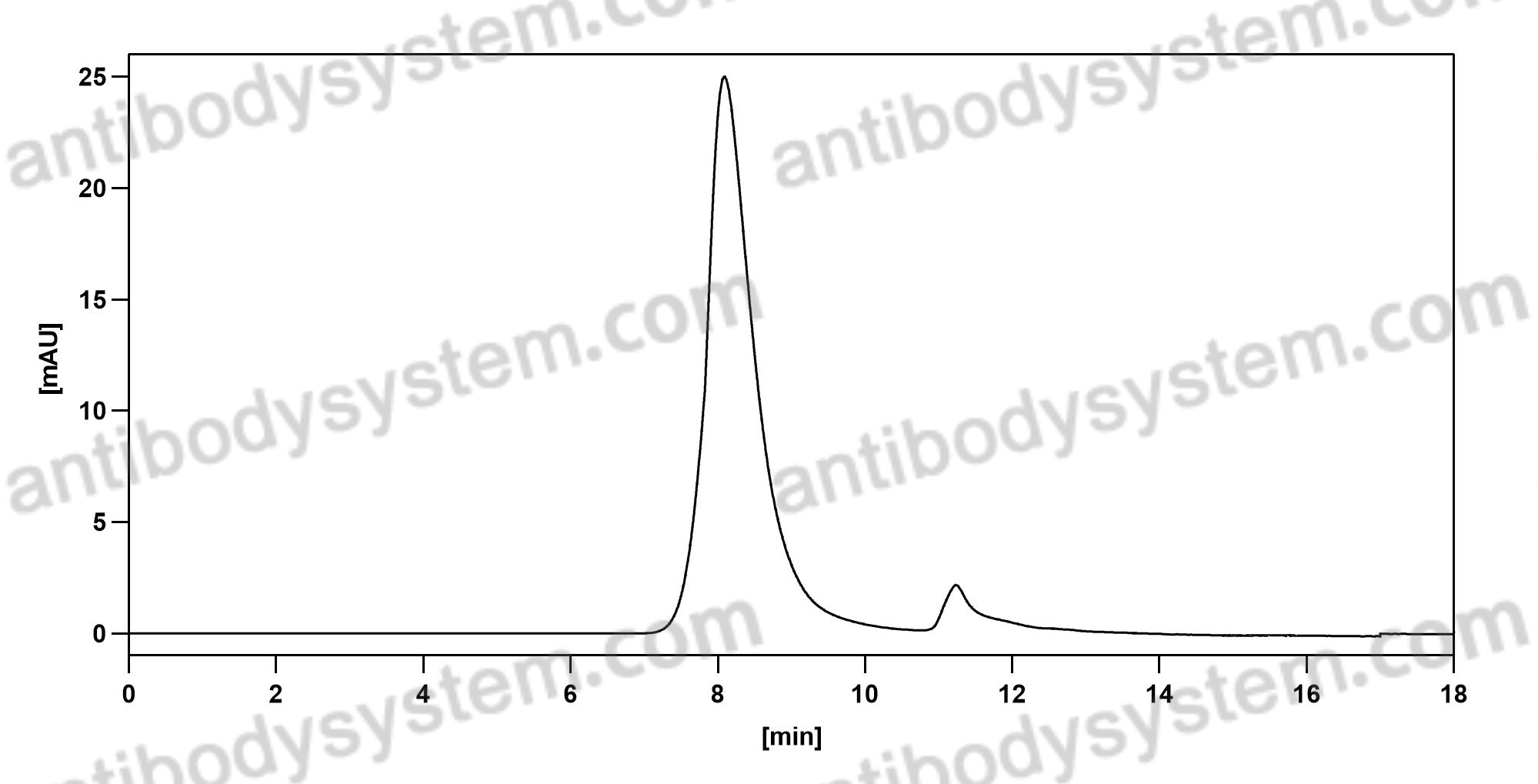
Natalizumab in Multiple Sclerosis: Long-Term Management, PMID: 28468254
Infection Risks Among Patients With Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies, PMID: 31589278
Natalizumab in Multiple Sclerosis Treatment: From Biological Effects to Immune Monitoring, PMID: 33072089
Natalizumab in acute ischemic stroke (ACTION II): A randomized, placebo-controlled trial, PMID: 32591475
Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing, PMID: 31515290
A review of the evidence for a natalizumab exit strategy for patients with multiple sclerosis, PMID: 30639651
Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP), PMID: 32234967
Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension, PMID: 29545067
Cancer Risk for Fingolimod, Natalizumab, and Rituximab in Multiple Sclerosis Patients, PMID: 32056253
Personalized extended interval dosing of natalizumab in MS: A prospective multicenter trial, PMID: 32690785
The risk of PML from natalizumab, PMID: 30784548
Natalizumab-Associated Primary Central Nervous System Lymphoma, PMID: 28962961
Natalizumab in relapsing-remitting multiple sclerosis, PMID: 27008031
A Randomized Trial Evaluating Various Administration Routes of Natalizumab in Multiple Sclerosis, PMID: 26835603
Natalizumab wearing-off effect: The hunt for the elusive pharmacodynamic biomarker, PMID: 32156774
Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study, PMID: 28209331
The role of natalizumab in the treatment of multiple sclerosis, PMID: 20615052
Use of natalizumab in multiple sclerosis: current perspectives, PMID: 27413840
Natalizumab treatment of multiple sclerosis: new insights, PMID: 28004598
Natalizumab for Multiple Sclerosis: A Case in Point for the Impact of Translational Neuroimmunology, PMID: 28167648
Natalizumab-associated progressive multifocal leukoencephalopathy in Germany, PMID: 30952796
Natalizumab: a new therapy for acute ischemic stroke?, PMID: 27476862
[Natalizumab treatment in multiple sclerosis], PMID: 26156254
Natalizumab induced cutaneous sarcoidosis-like reaction, PMID: 31108403
Natalizumab in secondary progressive multiple sclerosis, PMID: 29545068
Treatment of natalizumab-associated PML with filgrastim, PMID: 31139690
Pharmacokinetics and pharmacodynamics of natalizumab in pediatric patients with RRMS, PMID: 31355324
We know how to prescribe natalizumab for multiple sclerosis, but do we know how to withdraw it?, PMID: 24417188
Dosing interval of natalizumab in MS: Do good things come to those who wait?, PMID: 31515289
Natalizumab for induction of remission in Crohn's disease, PMID: 30068022
Extending the Interval of Natalizumab Dosing: Is Efficacy Preserved?, PMID: 31452081
Gynecological adverse effects of natalizumab administration: Case report and review of the literature, PMID: 30032043
Re-evaluating the incidence of natalizumab-associated progressive multifocal leukoencephalopathy, PMID: 27456891
Natalizumab-associated PML: Challenges with incidence, resulting risk, and risk stratification, PMID: 28228564
[Natalizumab], PMID: 26480703
Spanish consensus on the use of natalizumab (Tysabri®)-2013, PMID: 24360652
Natalizumab versus fingolimod for patients with active relapsing-remitting multiple sclerosis: results from REVEAL, a prospective, randomised head-to-head study, PMID: 33082194
Natalizumab is associated with early improvement of working ability in relapsing-remitting multiple sclerosis patients: WANT observational study results, PMID: 33205373
Exposure to natalizumab during pregnancy and lactation is safe - Commentary, PMID: 32508210
Exposure to natalizumab during pregnancy and lactation is safe - No, PMID: 32508200
Exposure to natalizumab during pregnancy and lactation is safe - Yes, PMID: 32508253
Pharmacodynamics of natalizumab extended interval dosing in MS, PMID: 32019876
Biomarkers identification for PML monitoring, during Natalizumab (Tysabri®) treatment in Relapsing-Remitting Multiple Sclerosis, PMID: 29353737
Clinical evaluation of natalizumab for formulary consideration, PMID: 20626228
Prediction of natalizumab anti-drug antibodies persistency, PMID: 29336205
Therapeutic drug monitoring of natalizumab, PMID: 30485149
Long-term safety evaluation of natalizumab for the treatment of multiple sclerosis, PMID: 28641055
Erythroblast appearance associated with natalizumab, PMID: 30711880
Effects of Natalizumab and Fingolimod on Clinical, Cognitive, and Magnetic Resonance Imaging Measures in Multiple Sclerosis, PMID: 31452082
Effectiveness and safety of natalizumab in real-world clinical practice: Review of observational studies, PMID: 27475049
Treatment Options for the Comorbidity of Multiple Sclerosis With Other Chronic Inflammatory Diseases., PMID:40526577
A comparison of JC virus assay performance provided with originator and biosimilar natalizumab., PMID:40515643
Beyond the switch to the biosimilar of natalizumab: What is the impact of changing the JCV test?, PMID:40515642
Diagnostic uncertainty in the era of biosimilar natalizumab-The case for harmonizing anti-JCV antibody testing., PMID:40515640
'Should natalizumab be used despite JC virus positivity? Yes'., PMID:40515591
Effectiveness of anti-CD20 therapies following natalizumab discontinuation: insights from a cohort study., PMID:40505538
What Is the Evidence on Immunomodulators and Immunosuppressants for Progressive Multiple Sclerosis? - A Cochrane Review Summary with Commentary., PMID:40485316
De-escalation of disease modifying therapies: A retrospective, observational single center study., PMID:40480031
The Italian Multiple Sclerosis Register Experience With Cladribine: Impact on Relapses, PIRA, and Treatment Sequencing Strategies Evaluation., PMID:40472290
A disproportionality analysis of nervous system adverse events associated with disease-modifying therapies in multiple sclerosis: insights from the FDA adverse event reporting system (FAERS)., PMID:40465056
Approach to JCV testing with natalizumab biosimilar: a UK consensus statement., PMID:40460616
Persistence to Ocrelizumab Compared with Other Disease-Modifying Therapies for Multiple Sclerosis: Results from the German NeuroTransData Registry., PMID:40455371
Comparative effectiveness of natalizumab and anti-CD20 monoclonal antibodies in relapsing-remitting multiple sclerosis: a real-world propensity-score matched study., PMID:40451284
Real-world evidence from Türkiye on cancer risk and treatment exposure in multiple sclerosis: A histopathology-verified, registry-based study., PMID:40449191
Should natalizumab be used despite JC virus positivity? No., PMID:40443199
Optimizing treatment for pediatric multiple sclerosis., PMID:40443188
Should natalizumab be used despite JC virus positivity?-Commentary., PMID:40443181
Real-World Treatment Outcomes in Black, Hispanic, Asian, and White Patients with Multiple Sclerosis Treated with Natalizumab., PMID:40442571
Correction: Pharmacogenomics of clinical response to Natalizumab in multiple sclerosis: a genome-wide multi-centric association study., PMID:40423827
Risk of stroke under disease modifying therapies for multiple sclerosis: a systematic review., PMID:40416416
Natalizumab-induced autoimmune liver injury., PMID:40414422
Natalizumab-induced autoimmune liver injury., PMID:40398815
Changes in circulating pro-inflammatory lymphocytes and cortical excitability with extended-interval natalizumab dosing in multiple sclerosis., PMID:40397308
Disease Course in Patients Switched from Natalizumab to Alemtuzumab: An Italian Multicenter, Prospective, Observational Study., PMID:40397068
Interventions promoting remyelination in multiple sclerosis: a systematic review of clinical trials., PMID:40393003
Comparative Efficacy and Safety of Extended Versus Standard Interval Dosing of Natalizumab in Relapsing-Remitting Multiple Sclerosis Patients: A Multicenter Analysis., PMID:40387571
Pediatric Multiple Sclerosis: A Systematic Exploration of Effectiveness in Current and Emerging Therapeutics., PMID:40381455
Comparison of CSF biomarkers in multiple sclerosis patients treated with natalizumab and rituximab., PMID:40345115
Long-term safety evaluation of natalizumab during pregnancy and lactation in patients with multiple sclerosis., PMID:40332701
Cost-effectiveness of ofatumumab for the treatment of relapsing forms of multiple sclerosis in the United Arab Emirates., PMID:40303316
Personalized treatment decision algorithms for the clinical application of serum neurofilament light chain in multiple sclerosis: A modified Delphi Study., PMID:40296363
Recrudescence of Natalizumab-Induced Pneumonitis and Peripheral Hypereosinophilia: Case Report and Literature Review., PMID:40292235
Selective IgM Hypogammaglobulinemia and Multiple Sclerosis Treated with Natalizumab and Ofatumumab: A Case Report., PMID:40278334
Impact of High-Efficacy Therapies for Multiple Sclerosis on B Cells., PMID:40277931
Should we stay or should we go? Recent insights on drug discontinuation in multiple sclerosis., PMID:40254626
Cost-Minimization Analysis and Budget Impact Analysis About Subcutaneous Natalizumab in Relapsing-Remitting Multiple Sclerosis in Italy., PMID:40236791
Generics, Biosimilars and Follow-On Non-Biologic Complex Drugs for Multiple Sclerosis: A Narrative Review of the Regulatory and Clinical Implications for European Neurologists., PMID:40231751
Serological screening and Vaccine Update in a cohort of Multiple Sclerosis Patients as a strategy to prevent infection reactivation during immunosuppressant therapy., PMID:40220724
Measuring cognitive change in secondary progressive MS: an analysis of the ASCEND cognition substudy., PMID:40220155
Impact of Natalizumab on Productivity and Ability to Work in Patients with Multiple Sclerosis in France: The TITAN Study., PMID:40208417
Comparing STRATIFY JCV™ DxSelect™ and IMMUNOWELL™ JCV IgG Tests in RRMS to Assess PML Risk., PMID:40197186
Acta neurologica belgica regarding the manuscript entitled 'Safety and efficacy of extended versus standard interval dosing of natalizumab in multiple sclerosis patients: a systematic review and meta-analysis'., PMID:40186744
Biosimilars versus biological therapy in inflammatory bowel disease: challenges and targeting strategies using drug delivery systems., PMID:40186719
Pyoderma gangrenosum in a patient with multiple sclerosis under natalizumab treatment: a case report., PMID:40175973
Trends in the real-world management of patients with active relapsing-remitting multiple sclerosis treated with natalizumab (TYSABRI®) in France: An analysis of the PMSI database over five years (2019-2023)., PMID:40169334
Accuracy of New John Cunningham Virus Antibody Assay in Natalizumab-Treated Patients With Multiple Sclerosis., PMID:40163140
Infection toxicity assessment of tumor necrosis factor α inhibitors in the treatment of IBD: a real-world study based on the US food and drug administration adverse events reporting system (FAERS)., PMID:40156444
No evidence of fluctuations in daily step count between infusions in people with multiple sclerosis treated with anti-CD20 monoclonal antibodies., PMID:40144903
Case Report: Ofatumumab treatment for concomitant multiple sclerosis and idiopathic thrombocytopenic purpura., PMID:40134425