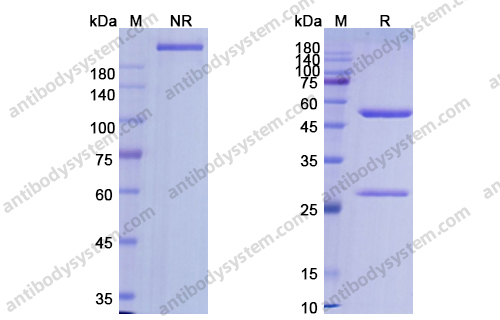
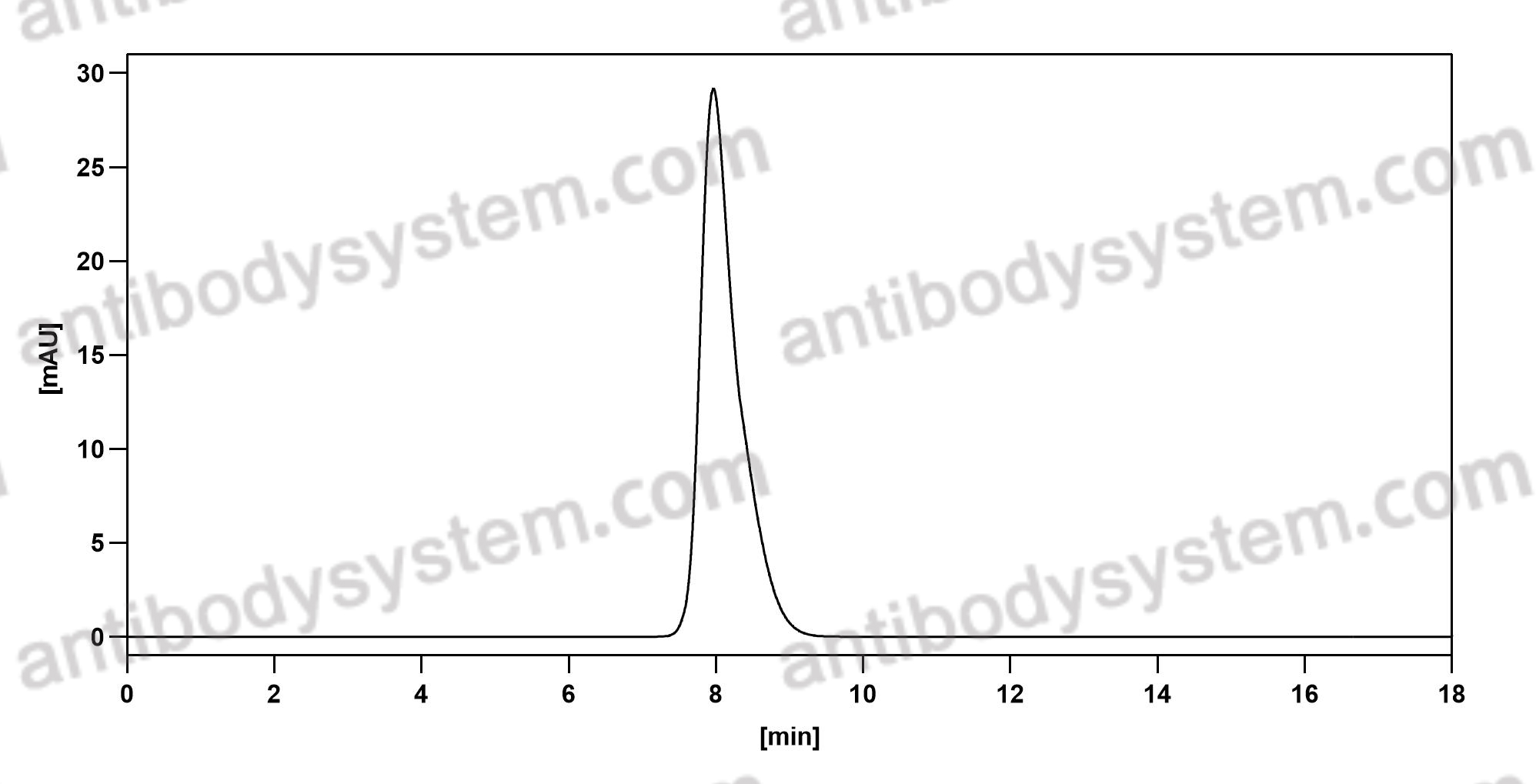
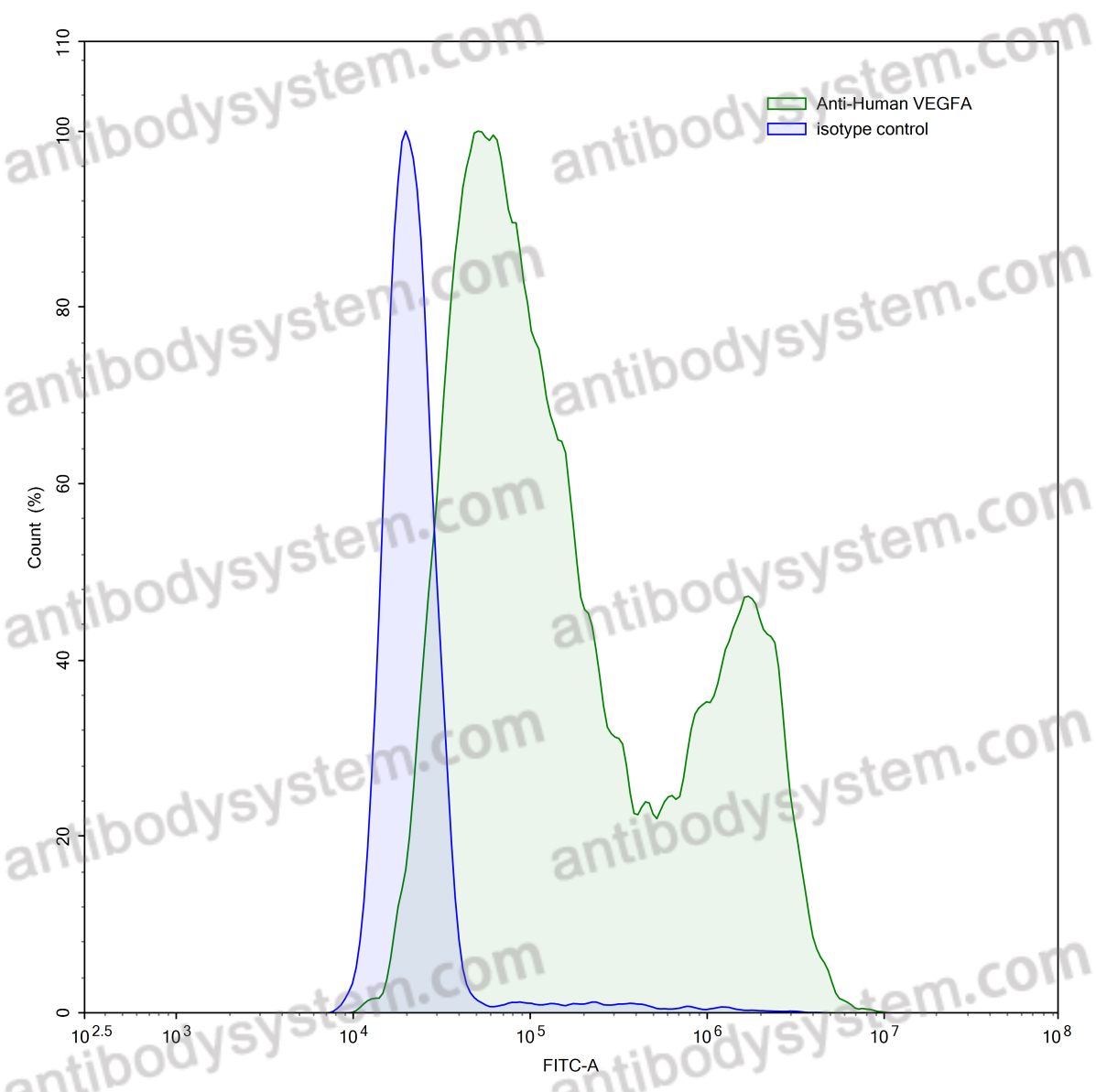
Alemtuzumab for the treatment of multiple sclerosis, PMID: 29309202
Alemtuzumab as Treatment for Multiple Sclerosis, PMID: 29500306
[Alemtuzumab therapy 2017], PMID: 29870645
Clinical pharmacology of alemtuzumab, an anti-CD52 immunomodulator, in multiple sclerosis, PMID: 30144037
The Meaning of Immune Reconstitution after Alemtuzumab Therapy in Multiple Sclerosis, PMID: 32503344
Alemtuzumab for Multiple Sclerosis, PMID: 27485945
A case of anaphylaxis to alemtuzumab, PMID: 30715557
Acquired haemophilia A after alemtuzumab therapy, PMID: 32700327
New cases of vasculitis after alemtuzumab, PMID: 32081067
Principles of alemtuzumab immunoablation in hematopoietic cell transplantation for non-malignant diseases in children: A review, PMID: 29352515
Alemtuzumab and prescription medication use in the MS population, PMID: 32403069
Alemtuzumab for multiple sclerosis, PMID: 27082500
Alemtuzumab therapy for multiple sclerosis, PMID: 23184314
Alemtuzumab, PMID: 18165760
Alemtuzumab Use in Clinical Practice: Recommendations from European Multiple Sclerosis Experts, PMID: 27882532
Alemtuzumab-associated diffuse alveolar damage - a case report, PMID: 32967641
Alemtuzumab in the treatment of multiple sclerosis: patient selection and special considerations, PMID: 27799738
Alemtuzumab therapy changes immunoglobulin levels in peripheral blood and CSF, PMID: 31826986
Anemia and sarcoidosis following treatment with alemtuzumab, PMID: 32979730
Alemtuzumab: Rare serious adverse events of a high-efficacy drug, PMID: 32298205
Alemtuzumab Induction Versus Conventional Immunosuppression in Heart Transplant Recipients, PMID: 31035777
Sarcoidosis following alemtuzumab treatment: Autoimmunity mediated by T cells and interferon-γ, PMID: 30307361
Alemtuzumab versus interferon beta 1a for relapsing-remitting multiple sclerosis, PMID: 29178444
Clinical observation during alemtuzumab administration, PMID: 32172992
Intracerebral haemorrhage during alemtuzumab administration, PMID: 30777657
Comparison of Alemtuzumab and Anti-thymocyte Globulin Treatment for Acute Kidney Allograft Rejection, PMID: 32719676
Alemtuzumab as rescue therapy in case of multiple sclerosis rebound following Natalizumab break: Clinical case and literature review, PMID: 30851640
Alemtuzumab for cytolytic induction of immunosuppression in heart transplant recipients, PMID: 23187050
A review on the treatment of sporadic inclusion body myositis with Bimagrumab and Alemtuzumab, PMID: 30238817
Alemtuzumab outcomes by age: Post hoc analysis from the randomized CARE-MS studies over 8 years, PMID: 33476880
Alemtuzumab induction therapy in kidney transplantation: a systematic review and meta-analysis, PMID: 22660659
Immune thrombocytopenia in alemtuzumab-treated MS patients: Incidence, detection, and management, PMID: 30785358
Listeria infection after treatment with alemtuzumab: a case report and literature review. Would antibiotic prophylaxis be considered?, PMID: 32487792
Alemtuzumab: the advantages and challenges of a novel therapy in MS, PMID: 24920854
Alemtuzumab to be restricted pending review, says EMA, PMID: 31034363
Efficacy and safety of alemtuzumab in Korean multiple sclerosis patients, PMID: 30849681
Pharmacokinetics of alemtuzumab in pediatric patients undergoing ex vivo T-cell-depleted haploidentical hematopoietic cell transplantation, PMID: 33037919
B and T cell prolymphocytic leukaemia, PMID: 31585622
Alemtuzumab-induced simultaneous onset of autoimmune haemolytic anaemia, alveolar haemorrhage, nephropathy, and stroke: A case report, PMID: 32446212
Emerging safety issues in alemtuzumab-treated MS patients, PMID: 31368417
Alemtuzumab as a Therapy for Chronic Lung Allograft Dysfunction in Lung Transplant Recipients With Short Telomeres, PMID: 32547557
Timing of Alemtuzumab With Respect to Day of Bone Marrow Infusion and its Effects Upon Engraftment and Graft-Versus-Host Disease in Patients With Sickle Cell Disease: A Single-Institutional Study, PMID: 32890079
Risk of opportunistic infections in patients treated with alemtuzumab for multiple sclerosis, PMID: 29848085
Kidney transplantation using alemtuzumab, belatacept, and sirolimus: Five-year follow-up, PMID: 32515087
Alemtuzumab depletion failure can occur in multiple sclerosis, PMID: 29247512
Alemtuzumab (Campath-1H) experience in kidney transplantation what we have learned; current practices; and scope for the future?, PMID: 26536426
The role of alemtuzumab in nonmyeloablative hematopoietic transplantation, PMID: 16720202
Monitoring and management of autoimmunity in multiple sclerosis patients treated with alemtuzumab: practical recommendations, PMID: 29525836
Alemtuzumab in Multiple Sclerosis: Short- and Long-Term Effects of Immunodepletion on the Peripheral Treg Compartment, PMID: 31214176
Overview of alemtuzumab therapy for the treatment of T-cell lymphomas, PMID: 21980956
Conditioning regimens for second allogeneic hematopoietic stem cell transplantation for patients with primary graft failure., PMID:40514014
Immunosuppressive agents in diabetes treatment: Hope or despair?, PMID:40487626
Potential use of the SARS-CoV-2 monoclonal antibody sipavibart in people with multiple sclerosis: definition of different patient archetypes from an Italian expert group perspective., PMID:40481170
The United Kingdom Hand and Upper Limb Transplantation Service: A report on the first twelve years of a single-center, single-protocol clinical cohort., PMID:40472654
A disproportionality analysis of nervous system adverse events associated with disease-modifying therapies in multiple sclerosis: insights from the FDA adverse event reporting system (FAERS)., PMID:40465056
Comparative analysis of five-year clinical outcomes of autologous hematopoietic stem cell transplantation and alemtuzumab in multiple sclerosis patients., PMID:40450828
Real-world evidence from Türkiye on cancer risk and treatment exposure in multiple sclerosis: A histopathology-verified, registry-based study., PMID:40449191
Combined Thymoglobulin and Interleukin-2 Receptor Antagonist Induction Therapy in Kidney Transplantation Is Associated With Worse Delayed Graft Function, Acute Rejection, Graft Loss, and Mortality., PMID:40440877
Clinical management of alemtuzumab-induced autoimmune thyroid diseases: a narrative review., PMID:40440332
Inefficiency Rates of Biological Immunosuppressive Induction Agents Used in Organ Transplantation: A Pharmacovigilance Study., PMID:40429403
Risk of stroke under disease modifying therapies for multiple sclerosis: a systematic review., PMID:40416416
Disease Course in Patients Switched from Natalizumab to Alemtuzumab: An Italian Multicenter, Prospective, Observational Study., PMID:40397068
Comparison of Regimens Used for Allogeneic Matched Related Donor Hematopoietic Cell Transplantation for Sickle Cell Disease., PMID:40379050
Alemtuzumab Exposure and T Lymphocyte Depletion: A Population Pharmacokinetic-Pharmacodynamic Model of Alemtuzumab Induction Therapy for Kidney Transplantation., PMID:40374867
Donor-specific mesenchymal stem cell infusion in human and nonhuman primate kidney transplantation., PMID:40368061
Identification of non-virologic risk factors for lymphoma after the first year of kidney transplant in adults: A retrospective analysis., PMID:40344959
Targeting EBV-infected T cells with alemtuzumab: a novel approach to systemic chronic active EBV disease., PMID:40304862
Impact of High-Efficacy Therapies for Multiple Sclerosis on B Cells., PMID:40277931
Effectiveness of Autologous Hematopoietic Stem Cell Transplantation versus Alemtuzumab and Ocrelizumab in Relapsing Multiple Sclerosis: A Single Center Cohort Study., PMID:40251896
Long-term outcomes and clinical phenotypes associated with best response to low dose alemtuzumab in cutaneous T-cell lymphoma., PMID:40246811
Baló's concentric sclerosis successfully treated with alemtuzumab: Long-term follow-up., PMID:40219936
Alemtuzumab for haematological malignancies., PMID:40214729
Treatment-resistant inflammatory demyelinating pseudotumor with Marburg-like features: A narrative review-based treatment approach., PMID:40200967
Real-world safety and effectiveness of alemtuzumab for relapsed or refractory chronic lymphocytic leukaemia: results from postmarketing surveillance in Japan., PMID:40156871
Therapeutic Advances in Pediatric Multiple Sclerosis., PMID:40150542
T-cell prolymphocytic leukemia, a case report and review of the literature., PMID:40109866
Assessment of the impact of reconstitution therapies-cladribine tablets and alemtuzumab-on the atrophy progression among patients with relapse-remitting multiple sclerosis., PMID:40084136
Herpes Zoster Infections With Multiple Sclerosis Disease-Modifying Therapies: A Real-World Pharmacovigilance Study., PMID:40083393
Systemic Targeted Therapies in Patients with Relapsed/Refractory Advanced Stage Cutaneous T-cell Lymphoma: A Real-world Single-centre Case Series., PMID:40079767
Rituximab for people with multiple sclerosis., PMID:40066932
[Comparing the efficacy of divozilimab and second-line treatments for relapsing-remitting multiple sclerosis in the Russian Federation: a systematic review and network meta-analysis]., PMID:40047834
Erratum to "Incorporation of alemtuzumab into frontline therapy of adult acute lymphoblastic leukemia: Results of Cancer and Leukemia Group B 10102 (Alliance), a phase 1/2 study"., PMID:40045671
Two Nonmyeloablative HLA-Matched Related Donor Allogeneic Hematopoietic Cell Transplantation Regimens in Patients with Severe Sickle Cell Disease., PMID:40010689
Cost-Utility Analysis and Efficiency Frontier of Drugs Available in Brazil for the Treatment of Relapsing-Remitting Multiple Sclerosis., PMID:39983504
Induction outcomes in adult kidney transplantation: Two decades of UNOS data analysis., PMID:39947488
Long-term efficacy and safety of alemtuzumab in participants with highly active MS: TOPAZ clinical trial and interim analysis of TREAT-MS real-world study., PMID:39935588
Evolving Patterns of Initial RRMS Treatment in Finland (2013-2022): Insights From a Nationwide Multiple Sclerosis Register., PMID:39935206
Relevance of Recent Thymic Emigrants Following Allogeneic Hematopoietic Cell Transplantation for Pediatric Patients with Inborn Errors of Immunity., PMID:39923938
Fatal pneumonia with repeated detection of Talaromyces columbinus two years after haploidentical transplantation., PMID:39922460
Results of Cancer and Leukemia Group B 10102 (Alliance), a Phase 1/2 Study., PMID:39916320
Comparison of outcomes following subcutaneous or intravenous alemtuzumab administered prior to reduced intensity conditioning for transplantation in pediatric sickle cell disease., PMID:39904467
Phase 2 study of alemtuzumab and dose-adjusted EPOCH-R in relapsed or refractory aggressive B-cell lymphomas., PMID:39899393
Alemtuzumab and thrombotic thrombocytopenic purpura: Analysis of an international surveillance database and systematic literature review., PMID:39883995
Multicentre adaptive randomised trial of GvHD prophylaxis following unrelated donor stem cell transplantation comparing Thymoglobulin versus calcineurin inhibitor-based or sirolimus-based post-transplant cyclophosphamide (Methods of T cell Depletion, MoTD trial)., PMID:39880444
Fingolimod and risk of skin cancer among individuals with multiple sclerosis: a population-based cohort study protocol., PMID:39855661
Effect of Different Treatments on Retinal Thickness Changes in Patients With Multiple Sclerosis: A Review., PMID:39853938
The Many Faces of Philadelphia: A Mature T-Cell Lymphoma with Variant Philadelphia-Translocation and Duplication of the Philadelphia Chromosome., PMID:39846605
The immunological bases of alemtuzumab as induction-therapy in pediatric-onset multiple sclerosis., PMID:39845956
Efficacy and safety of disease-modifying therapies in pediatric-onset multiple sclerosis: A systematic review of clinical trials and observational studies., PMID:39805178
Proteasome Inhibitors Induce Apoptosis in Ex Vivo Cells of T-Cell Prolymphocytic Leukemia., PMID:39769335