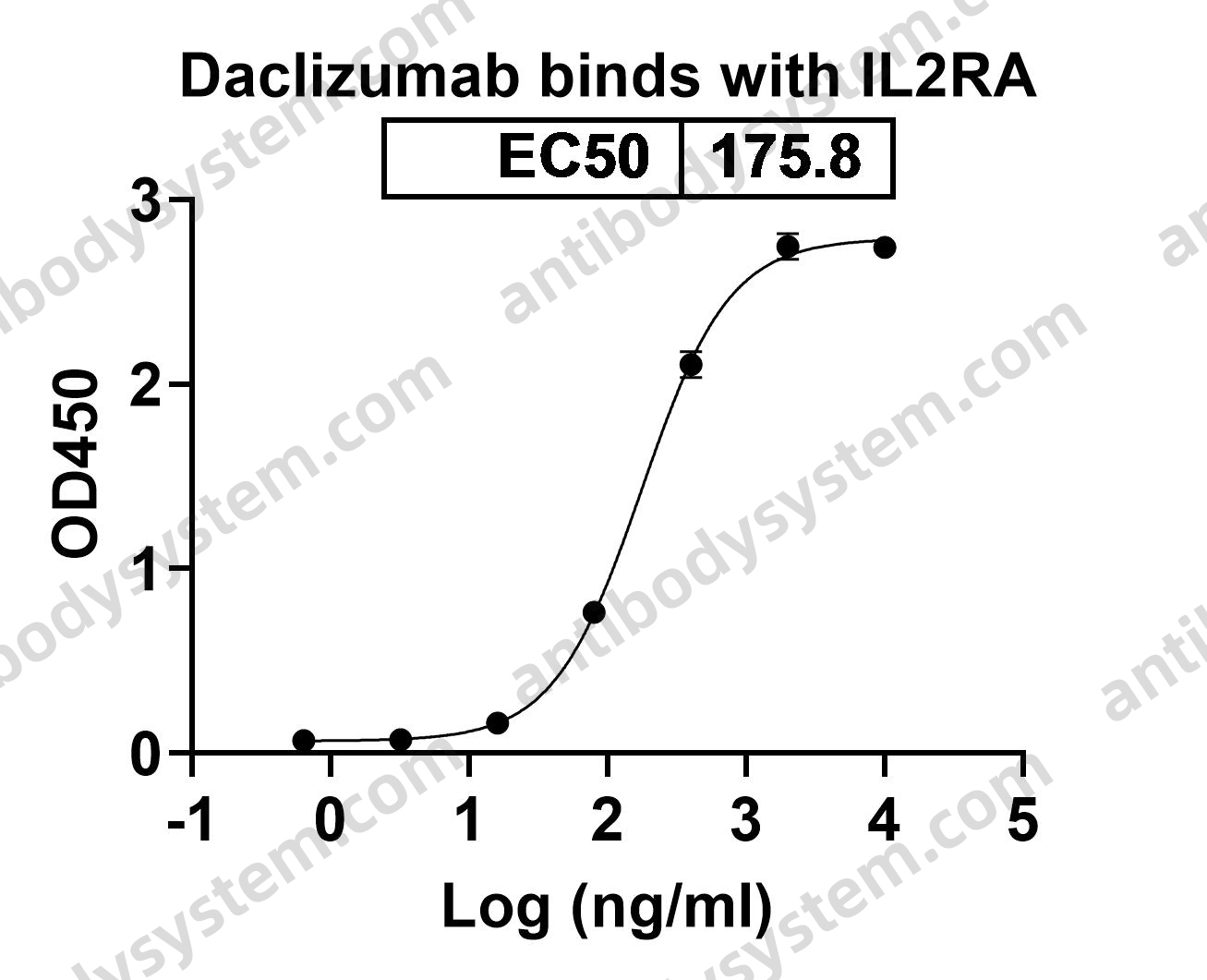
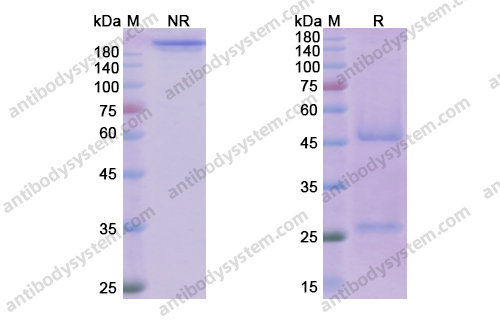
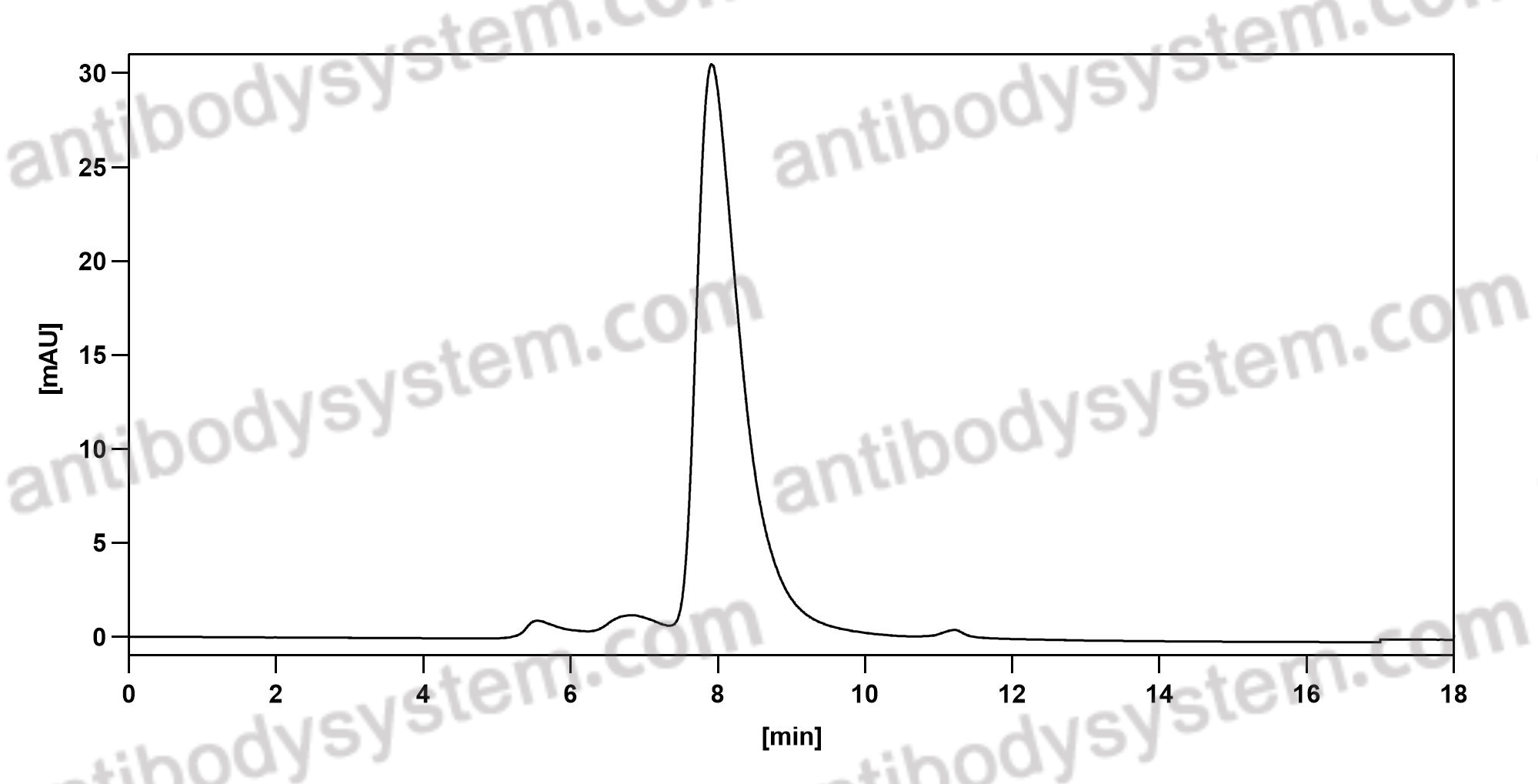
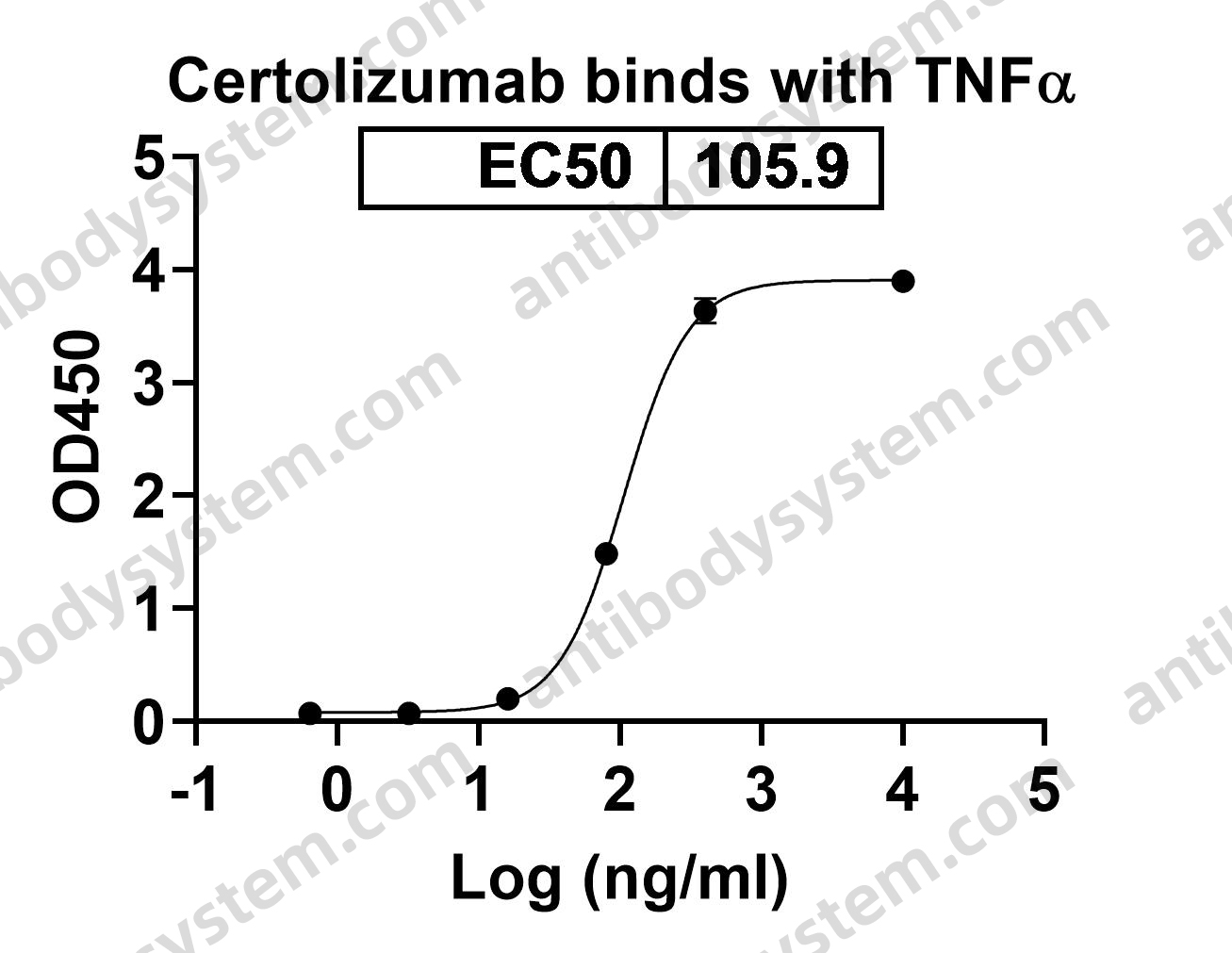
Daclizumab Therapy for Multiple Sclerosis, PMID: 29661806
Daclizumab in multiple sclerosis, PMID: 29645071
▼ Daclizumab for MS, PMID: 28183723
Daclizumab, PMID: 17916050
Daclizumab: A Review in Relapsing Multiple Sclerosis, PMID: 28211007
Daclizumab for the treatment of multiple sclerosis, PMID: 28849702
Daclizumab for the treatment of adults with relapsing forms of multiple sclerosis, PMID: 28803486
Daclizumab therapy for multiple sclerosis, PMID: 23055048
Daclizumab-induced encephalitis in multiple sclerosis, PMID: 31549941
Daclizumab: Development, Clinical Trials, and Practical Aspects of Use in Multiple Sclerosis, PMID: 28707278
Daclizumab and its use in multiple sclerosis treatment, PMID: 28387383
Daclizumab for relapsing remitting multiple sclerosis, PMID: 22513956
Pharmacokinetic drug evaluation of daclizumab for the treatment of relapsing-remitting multiple sclerosis, PMID: 29363337
Daclizumab for relapsing remitting multiple sclerosis, PMID: 24363032
Daclizumab for relapsing remitting multiple sclerosis, PMID: 20556791
Daclizumab (anti-CD25) in multiple sclerosis, PMID: 24768797
Daclizumab: a review of its use in the management of organ transplantation, PMID: 11707149
Daclizumab: a potential asthma therapy?, PMID: 20804450
Daclizumab (anti-Tac, Zenapax) in the treatment of leukemia/lymphoma, PMID: 17530023
Daclizumab: a review of its use in the prevention of acute rejection in renal transplant recipients, PMID: 10651389
Modulation of IL-2Rα with daclizumab for treatment of multiple sclerosis, PMID: 23732529
Characterization of the Impact of Daclizumab Beta on Circulating Natural Killer Cells by Mass Cytometry, PMID: 32391016
Immune-mediated encephalitis with daclizumab: The final nail, PMID: 30073908
End of the road for daclizumab in multiple sclerosis, PMID: 29565004
Severe meningo-/encephalitis after daclizumab therapy for multiple sclerosis, PMID: 30657420
A critical appraisal of daclizumab use as emerging therapy in multiple sclerosis, PMID: 25826609
Review of daclizumab and its therapeutic potential in the treatment of relapsing-remitting multiple sclerosis, PMID: 24143075
Long-term safety and efficacy of daclizumab beta in relapsing-remitting multiple sclerosis: 6-year results from the SELECTED open-label extension study, PMID: 32451615
Anti-CD25 (daclizumab) monoclonal antibody therapy in relapsing-remitting multiple sclerosis, PMID: 22284868
Daclizumab for the treatment of relapsing-remitting multiple sclerosis, PMID: 28286970
Daclizumab to prevent rejection after cardiac transplantation, PMID: 15987919
90 Y-Daclizumab (Anti-CD25), High-Dose Carmustine, Etoposide, Cytarabine, and Melphalan Chemotherapy and Autologous Hematopoietic Stem Cell Transplant Yielded Sustained Complete Remissions in 4 Patients with Recurrent Hodgkin's Lymphoma, PMID: 32275165
Daclizumab HYP versus Interferon Beta-1a in Relapsing Multiple Sclerosis, PMID: 26444729
A fatal case of daclizumab-induced liver failure in a patient with MS, PMID: 30800722
Daclizumab (Zinbryta) for multiple sclerosis, PMID: 27603962
The development of sarcoidosis in patients receiving daclizumab: A case series from multiple clinical trials, PMID: 30885425
Disease modifying therapies for relapsing multiple sclerosis, PMID: 27549763
Anti-interleukin-2 receptor antibodies: basiliximab and daclizumab, PMID: 11522853
Daclizumab use in patients with pediatric multiple sclerosis, PMID: 22232346
Rash developing after cessation of Daclizumab for relapsing remitting MS; a case series, PMID: 31421627
Daclizumab-induced adverse events in multiple organ systems in multiple sclerosis, PMID: 24532277
Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial, PMID: 23562009
Severe cutaneous adverse event in a daclizumab-treated multiple sclerosis patient, PMID: 31228714
Delayed onset hypophysitis after therapy with daclizumab for multiple sclerosis - A report of two cases, PMID: 33387829
Daclizumab induction therapy in liver transplant recipients with renal insufficiency, PMID: 20659283
Daclizumab for treatment of birdshot chorioretinopathy, PMID: 18268208
[Off-label use of biologic agents in the treatment of dermatosis, part 2: etanercept, efalizumab, alefacept, rituximab, daclizumab, basiliximab, omalizumab, and cetuximab], PMID: 18206084
Spotlight on anti-CD25: daclizumab in MS, PMID: 18808743
Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): a multicentre, randomised, double-blind extension trial, PMID: 24656609
A case of immune-mediated encephalitis related to daclizumab therapy, PMID: 30073905
Induction With Antithymocyte Globulin Is Associated With Decreased Mortality and PTLD in Pediatric Liver Transplantation: A UNOS Data Analysis., PMID:40490923
A peek into the pipeline: Expert views on emerging therapies., PMID:40239631
T-bet+ CXCR3+ B cells drive hyperreactive B-T cell interactions in multiple sclerosis., PMID:40107244
Evolving Patterns of Initial RRMS Treatment in Finland (2013-2022): Insights From a Nationwide Multiple Sclerosis Register., PMID:39935206
Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis and neuromyelitis optica spectrum disorder - recommendations from ECTRIMS and the EBMT., PMID:39814869
Identifying Promising Immunomodulators for Type 1 Diabetes (T1D) and Islet Transplantation., PMID:39735417
Intrathecal immune reactivity against Measles-, Rubella-, and Varicella Zoster viruses is associated with cerebrospinal fluid inflammation in multiple sclerosis., PMID:39377663
Immunomodulators and immunosuppressants for progressive multiple sclerosis: a network meta-analysis., PMID:39254048
Autoantigen-specific CD4+ T cells acquire an exhausted phenotype and persist in human antigen-specific autoimmune diseases., PMID:39226901
Exploration of the Shared Gene Signatures and Molecular Mechanisms between Chronic Bronchitis and Antineutrophil Cytoplasmic Antibody-associated Glomerulonephritis: Evidence from Transcriptome Data., PMID:38847168
Alterations of Thymus-Derived Tregs in Multiple Sclerosis., PMID:38838284
Induction therapy in heart transplantation: A systematic review and network meta-analysis for developing evidence-based recommendations., PMID:38716786
Interleukin inhibitors and the associated risk of candidiasis., PMID:38605952
Disease-modifying therapies in managing disability worsening in paediatric-onset multiple sclerosis: a longitudinal analysis of global and national registries., PMID:38547883
The Effect of Induction Therapy on Antibody-Mediated Rejection in Kidney Transplantation: A Network Meta-Analysis Using Recent Data., PMID:38490831
Drug-Induced Progressive Multifocal Leukoencephalopathy (PML): A Systematic Review and Meta-Analysis., PMID:38321317
Non-IL-2-blocking anti-CD25 antibody inhibits tumor growth by depleting Tregs and has synergistic effects with anti-CTLA-4 therapy., PMID:38180065
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis., PMID:38174776
Outcomes of kidney transplantation in patients with IgA nephropathy based on induction: A UNOS data analysis., PMID:38127110
Adverse effects of immunotherapies for multiple sclerosis: a network meta-analysis., PMID:38032059
Network meta-analysis on efficacy and safety of different biologics for ulcerative colitis., PMID:37803294
"No association between disease modifying treatment and fatigue in multiple sclerosis"., PMID:37708819
A genome-wide association study of blood cell morphology identifies cellular proteins implicated in disease aetiology., PMID:37596262
A Real-World Disproportionality Analysis of Drug-Induced Immune Hemolytic Anemia in the FDA Adverse Event Reporting System., PMID:37522435
Treatment with Biologic Drugs in Pediatric Behçet's Disease: A Comprehensive Analysis of the Published Data., PMID:37382804
Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma., PMID:37198490
A first case of successful using of ibrutinib in treating paraneoplastic pemphigus related bronchiolitis obliterans concurrent with CLL., PMID:37007770
Seizure risk in multiple sclerosis patients treated with disease-modifying therapy: A systematic review and network meta-analysis., PMID:36802988
Dynamics of Inflammatory and Neurodegenerative Biomarkers after Autologous Hematopoietic Stem Cell Transplantation in Multiple Sclerosis., PMID:36142860
What Have Failed, Interrupted, and Withdrawn Antibody Therapies in Multiple Sclerosis Taught Us?, PMID:35794296
Novel multiple sclerosis agents-associated cardiotoxicity: A real-world pharmacovigilance study., PMID:35643216
Long-term efficacy and safety of siponimod in patients with secondary progressive multiple sclerosis: Analysis of EXPAND core and extension data up to >5 years., PMID:35380078
The Shared Mechanism and Candidate Drugs of Multiple Sclerosis and Sjögren's Syndrome Analyzed by Bioinformatics Based on GWAS and Transcriptome Data., PMID:35356004
Decreased Intrathecal Concentrations of Free Light Chains Kappa in Multiple Sclerosis Patients Taking Very High Effective Disease-Modifying Treatment., PMID:35328273
Current Knowledge of Biologics in Treatment of Noninfectious Uveitis., PMID:35133890
Trends in the use of disease-modifying therapies among reproductive-aged women with multiple sclerosis in the United States from 2010 to 2019., PMID:35088492
Mechanistic and Biomarker Studies to Demonstrate Immune Tolerance in Multiple Sclerosis., PMID:35069562
NK Cells and Innate-Like T Cells After Autologous Hematopoietic Stem Cell Transplantation in Multiple Sclerosis., PMID:34975899
Antibody-guided design and identification of CD25-binding small antibody mimetics using mammalian cell surface display., PMID:34764369
Meta-Analysis of Interleukin-2 Receptor Antagonists as the Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease., PMID:34621279
Biologic Therapies and Small Molecules for the Management of Non-Infectious Scleritis: A Narrative Review., PMID:34476773
Correction: Bortezomib for anti-NMDAR encephalitis following daclizumab treatment in a patient with multiple sclerosis., PMID:34396130
Maintenance treatment with infliximab for ulcerative ileitis after intestinal transplantation: A case report., PMID:34307578
Bortezomib for anti-NMDAR encephalitis following daclizumab treatment in a patient with multiple sclerosis., PMID:34079936
Impact of Interferons and Biological Drug Inhibitors of IL-2 and IL-6 on Small-Molecule Drug Metabolism Through the Cytochrome P450 System., PMID:34078115
A Network Meta-Analysis of Induction Immunosuppression for Simultaneous Pancreas-Kidney Transplant From Randomized Clinical Trials., PMID:34053419
Multiple sclerosis: doubling down on MHC., PMID:34006391