Catalog No.
RHB94401
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
TNF, Tumor necrosis factor ligand superfamily member 2, N-terminal fragment, ICD2, NTF, TNF-a, TNF-alpha, Tumor necrosis factor, TNFSF2, TNFA, Cachectin, ICD1
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
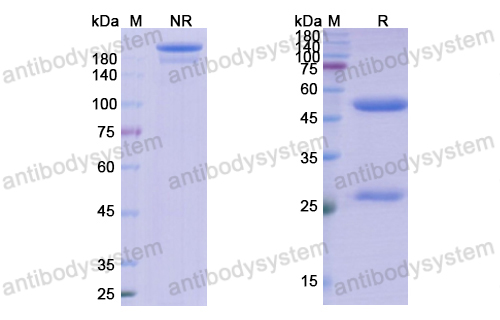
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P01375
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Clone ID
CDP571
Apheresis: A cell-based therapeutic tool for the inflammatory bowel disease., PMID:36158031
Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease., PMID:26896086
Biologic targeting in the treatment of inflammatory bowel diseases., PMID:19707398
TNF-alpha and its inhibitors in cancer., PMID:19277912
Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn's disease., PMID:18254120
Managing Crohn disease in children and adolescents : focus on tumor necrosis factor antagonists., PMID:18162006
Biological agents in rheumatoid arthritis: which ones could be used in combination?, PMID:18020567
Current and Future Anti-TNF Therapy for Inflammatory Bowel Disease., PMID:17547858
Do anti-tumor necrosis factors induce response and remission in patients with acute refractory Crohn's disease? A systematic meta-analysis of controlled clinical trials., PMID:17184965
Biological therapies for inflammatory bowel disease: research drives clinics., PMID:16842127
What's new: innovative concepts in inflammatory bowel disease., PMID:16594957
Drug insight: antagonists of tumor-necrosis factor-alpha in the treatment of inflammatory bowel disease., PMID:16582964
CDP571, a humanized monoclonal antibody to tumour necrosis factor-alpha, for steroid-dependent Crohn's disease: a randomized, double-blind, placebo-controlled trial., PMID:16480401
Biological therapies in IBD: an international course., PMID:16025380
Management of Perianal Crohn's Disease., PMID:15913509
New concepts in anti-tumor necrosis factor therapy for inflammatory bowel disease., PMID:15741928
A randomized, double-blind, placebo-controlled trial of CDP571, a humanized monoclonal antibody to tumour necrosis factor-alpha, in patients with corticosteroid-dependent Crohn's disease., PMID:15709987
Anti-TNF therapy in Crohn's disease., PMID:15669643
CDP571, a humanized anti-tumor necrosis factor-alpha monoclonal antibody in pediatric Crohn's disease., PMID:15626889
CDP571, a humanised monoclonal antibody to tumour necrosis factor alpha, for moderate to severe Crohn's disease: a randomised, double blind, placebo controlled trial., PMID:15361500
Newer Therapies for Inflammatory Bowel Disease., PMID:15149578
The use of TNF-alpha blocking agents in rheumatoid arthritis: an overview., PMID:15013927
Tumor necrosis factor-alpha antibody for induction of remission in Crohn's disease., PMID:14974022
Optimizing anti-tumor necrosis factor strategies in inflammatory bowel disease., PMID:14602060
Gateways to clinical trials., PMID:12851663
Review article: maintenance treatment of Crohn's disease., PMID:12786610
Strategies for targeting tumour necrosis factor in IBD., PMID:12617886
Gateways to clinical trials., PMID:12616965
Therapeutic inhibitors of tumor necrosis factor in Crohn's disease., PMID:12498004
Corticosteroid-sparing treatments in patients with Crohn's disease., PMID:12135008
Biologic therapy of inflammatory bowel disease., PMID:12016425
[Treatment of Crohn's disease by anti-cytokine monoclonal antibodies]., PMID:11808321
Managing the glucocorticoid dependent inflammatory bowel disease patient., PMID:11726077
Evolving medical therapies for Crohn's disease., PMID:11696284
Strategies targeting tumor necrosis factor in Crohn's disease., PMID:11475128
Possible role of TNF on procalcitonin release in a baboon model of sepsis., PMID:11442311
Transcending conventional therapies: the role of biologic and other novel therapies., PMID:11380043
Approach to corticosteroid-dependent and corticosteroid-refractory Crohn's disease., PMID:11380040
An engineered human antibody to TNF (CDP571) for active Crohn's disease: a randomized double-blind placebo-controlled trial., PMID:11313302
TNF-alpha antagonists for the treatment of Crohn's disease., PMID:11249506
[Value of anti-TNF-alpha molecules in inflammatory and infectious diseases]., PMID:11075396
Steroid-dependent Crohn's disease., PMID:11023556
The engineered human anti-tumor necrosis factor-alpha antibody CDP571 inhibits inflammatory pathways but not T cell activation in patients with rheumatoid arthritis., PMID:10555883
New therapeutic targets for rheumatoid arthritis., PMID:10380231
Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety., PMID:10338381
Combination therapy of pentoxifylline and TNFalpha monoclonal antibody in dextran sulphate-induced mouse colitis., PMID:10102957
The future role of anti-tumour necrosis factor-alpha products in the treatment of Crohn's disease., PMID:9777308
Treatment of ulcerative colitis with an engineered human anti-TNFalpha antibody CDP571., PMID:9663825
Treatment of ulcerative colitis in the cottontop tamarin using antibody to tumour necrosis factor alpha., PMID:9203942
Randomised controlled trial of CDP571 antibody to tumour necrosis factor-alpha in Crohn's disease., PMID:9048788