Catalog No.
DHC43302
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Pancreatic carcinoma marker protein GA733-1, Tumor-associated calcium signal transducer 2, M1S1, TROP2, TACSTD2, Cell surface glycoprotein Trop-2, GA733-1, Membrane component chromosome 1 surface marker 1
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
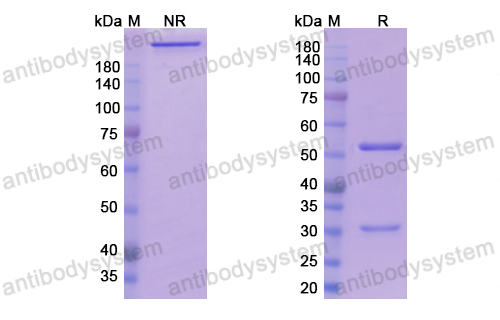
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P09758
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
DS-1062, DS-1062a, Datopotamab deruxtecan, CAS: 2267989-53-5
Clone ID
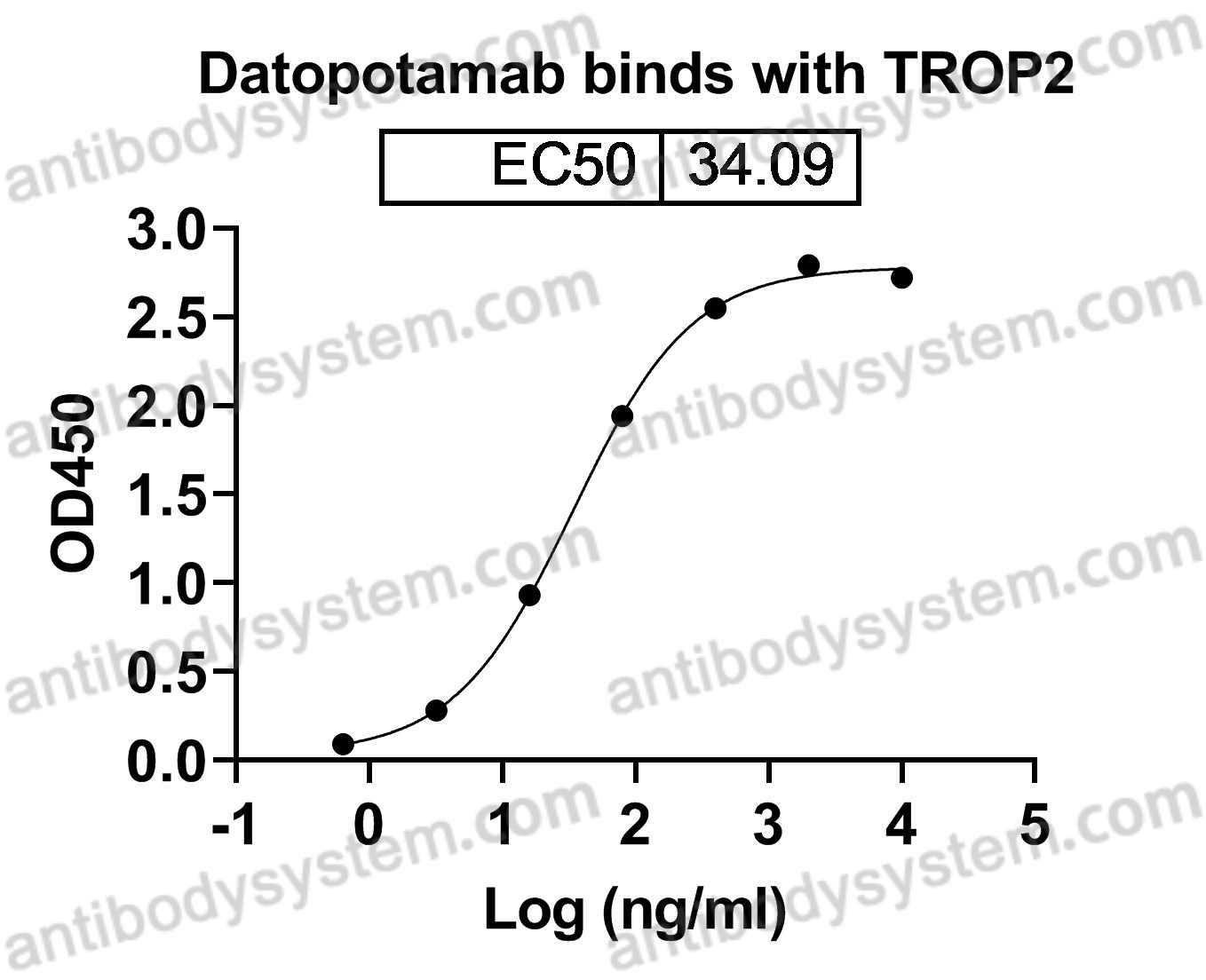
Datopotamab
Datopotamab deruxtecan (Dato-DXd), a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells, PMID: 34413126
TROP2 ADC Intrigues in NSCLC, PMID: 33597275
A pooled analysis of datopotamab deruxtecan in patients with EGFR-mutated NSCLC., PMID:40516821
An evaluation of datopotamab deruxtecan for the treatment of non-small cell lung cancer., PMID:40515573
Efficacy and Safety of TROP-2-Targeting Antibody-Drug Conjugate Treatment in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Pooled Analysis of Reconstructed Patient Data., PMID:40507234
Synergistic strategies: ADC-PARP inhibitor combinations in triple-negative breast cancer therapy., PMID:40494034
Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trial., PMID:40450142
Efficacy and Safety of Antibody-Drug Conjugates for Lung Cancer Therapy: A Systematic Review of Randomized and Non-Randomized Clinical Trials., PMID:40430899
Cost-effectiveness of datopotamab deruxtecan in previously treated advanced nonsquamous NSCLC., PMID:40388791
Decoding Clinical Trials in Metastatic Breast Cancer: Practical Insights for Optimal Therapy Sequencing., PMID:40367401
Datopotamab Deruxtecan: First Approval., PMID:40323341
Preclinical Activity of Datopotamab Deruxtecan, an Antibody-Drug Conjugate Targeting Trophoblast Cell-Surface Antigen 2, in Uterine Serous Carcinoma., PMID:40299780
TROPION-Breast05: a randomized phase III study of Dato-DXd with or without durvalumab versus chemotherapy plus pembrolizumab in patients with PD-L1-high locally recurrent inoperable or metastatic triple-negative breast cancer., PMID:40297626
Therapeutic Potential of Datopotamab Deruxtecan in the Treatment of Advanced Non-Small Cell Lung Cancer: Evidence to Date., PMID:40291609
[Diffuse interstitial lung disease induced by antibody-drug conjugates]., PMID:40263022
Targeting Lung Cancer with Precision: The ADC Therapeutic Revolution., PMID:40238068
FDA approves Datroway: a novel therapy for HR-positive, HER2-negative metastatic breast cancer., PMID:40212175
[Standard of care of EGFR mutated metastatic NSCLC in first treatment and beyond progression]., PMID:40155080
Reply to: Assessing Clinical Utility of Datopotamab Deruxtecan Versus Chemotherapy for Breast Cancer., PMID:40153734
Assessing Clinical Utility of Datopotamab Deruxtecan Versus Chemotherapy for Breast Cancer., PMID:40153615
Prophylaxis, clinical management, and monitoring of datopotamab deruxtecan-associated oral mucositis/stomatitis., PMID:40139260
Antibody-drug conjugates in breast cancer: current evidence and future directions., PMID:40114224
Preclinical evaluation of a novel antibody-drug conjugate OBI-992 for Cancer therapy., PMID:40082588
Antibody-drug conjugates in elderly patients with breast cancer., PMID:40020509
Datopotamab deruxtecan (Datroway) for advanced breast cancer., PMID:40009990
TROPION-Breast04: a randomized phase III study of neoadjuvant datopotamab deruxtecan (Dato-DXd) plus durvalumab followed by adjuvant durvalumab versus standard of care in patients with treatment-naïve early-stage triple negative or HR-low/HER2- breast cancer., PMID:39917260
Beyond failure of endocrine-based therapies in HR+/HER2 negative advanced breast cancer: What before chemotherapy? A glimpse into the future., PMID:39900320
Management of nausea and vomiting induced by antibody-drug conjugates., PMID:39878905
OBI-992, a Novel TROP2-Targeted Antibody-Drug Conjugate, Demonstrates Antitumor Activity in Multiple Cancer Models., PMID:39786401
Datopotamab Deruxtecan in Advanced or Metastatic Non-Small Cell Lung Cancer With Actionable Genomic Alterations: Results From the Phase II TROPION-Lung05 Study., PMID:39761483
Antibodies to watch in 2025., PMID:39711140
Efficacy and safety of antibody-drug conjugates in pretreated HER2-low metastatic breast cancer: A systematic review and network meta-analysis., PMID:39709655
Emerging Therapies for Brain Metastases in NSCLC, Breast Cancer, and Melanoma: A Critical Review., PMID:39625633
Antitumor Activity and Biomarker Analysis for TROP2 Antibody-Drug Conjugate Datopotamab Deruxtecan in Patient-Derived Breast Cancer Xenograft Models., PMID:39585341
Advances in Trop-2 targeted antibody-drug conjugates for breast cancer: mechanisms, clinical applications, and future directions., PMID:39555080
TROPION-Lung07: Phase III study of Dato-DXd + pembrolizumab ± platinum-based chemotherapy as 1L therapy for advanced non-small-cell lung cancer., PMID:39469838
Understanding the chemistry & pharmacology of antibody-drug conjugates in triple-negative breast cancer with special reference to exatecan derivatives., PMID:39460856
Can We Find a Place for Trophoblast Cell Surface Antigen 2-Targeted Antibody-Drug Conjugates in Lung Cancer?, PMID:39353165
Datopotamab-deruxtecan plus durvalumab in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial., PMID:39277672
Datopotamab-deruxtecan in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial., PMID:39277671
Datopotamab Deruxtecan Versus Chemotherapy in Previously Treated Inoperable/Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer: Primary Results From TROPION-Breast01., PMID:39265124
Datopotamab Deruxtecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non-Small Cell Lung Cancer: The Randomized, Open-Label Phase III TROPION-Lung01 Study., PMID:39250535
Preclinical activity of datopotamab deruxtecan, a novel TROP2 directed antibody-drug conjugate targeting trophoblast cell-surface antigen 2 (TROP2) in ovarian carcinoma., PMID:38981151
Blockade of SIRPα-CD47 axis by anti-SIRPα antibody enhances anti-tumor activity of DXd antibody-drug conjugates., PMID:38843278
TROPION-Breast03: a randomized phase III global trial of datopotamab deruxtecan ± durvalumab in patients with triple-negative breast cancer and residual invasive disease at surgical resection after neoadjuvant therapy., PMID:38686016
Perspectives on geriatric oncology research presented at the 2023 San Antonio Breast Cancer Symposium: A Young International Society of Geriatric Oncology report., PMID:38677935
Datopotamab Deruxtecan in Advanced or Metastatic HR+/HER2- and Triple-Negative Breast Cancer: Results From the Phase I TROPION-PanTumor01 Study., PMID:38652877
Antibody-drug conjugates in lung and breast cancer: current evidence and future directions-a position statement from the ETOP IBCSG Partners Foundation., PMID:38648979
Datopotamab deruxtecan: A novel antibody drug conjugate for triple-negative breast cancer., PMID:38560142
Clinical management, monitoring, and prophylaxis of adverse events of special interest associated with datopotamab deruxtecan., PMID:38502995
Best of the year: Advanced breast cancer in 2023., PMID:38401422