Catalog No.
DHC65501
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
SAA, Serum amyloid A-1 protein, SAA1, Amyloid fibril protein AA
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
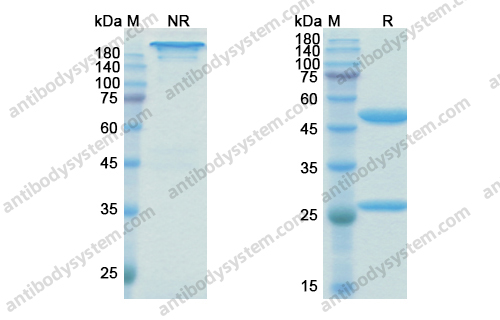
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P0DJI8
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
2A4, ELT1-01, NEOD-001, CAS: 1608108-91-3
Clone ID
Birtamimab
Novel Approaches for the Management of AL Amyloidosis, PMID: 29951831
Beyond NEOD001 for systemic light-chain amyloidosis, PMID: 30242086
Market watch: Upcoming market catalysts in Q2 2018, PMID: 29588523
First-in-Human Phase I/II Study of NEOD001 in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction, PMID: 26858336
Organ response in patients with AL amyloidosis treated with NEOD001, an amyloid-directed monoclonal antibody, PMID: 27648922
Etiological Treatment of Cardiac Amyloidosis: Standard of Care and Future Directions., PMID:40232627
New therapies to treat cardiac amyloidosis., PMID:39819772
Current and Emerging Immunotherapies for Systemic AL Amyloidosis., PMID:39327239
The mechanism of action, pharmacological characteristics, and clinical utility of the amyloid depleter birtamimab for the potential treatment of AL amyloidosis., PMID:38600883
Novel monoclonal antibodies: A really specific therapy for light chain amyloidosis., PMID:38590272
Birtamimab: a new amyloidosis treatment?, PMID:37796521
Birtamimab plus standard of care in light-chain amyloidosis: the phase 3 randomized placebo-controlled VITAL trial., PMID:37366170
Advancements and future trends of immunotherapy in light-chain amyloidosis., PMID:36696931
Multidisciplinary supportive care in systemic light chain amyloidosis., PMID:35593003
Beyond NEOD001 for systemic light-chain amyloidosis., PMID:30242086
Novel Approaches for the Management of AL Amyloidosis., PMID:29951831
Market watch: Upcoming market catalysts in Q2 2018., PMID:29588523
Organ response in patients with AL amyloidosis treated with NEOD001, an amyloid-directed monoclonal antibody., PMID:27648922
First-in-Human Phase I/II Study of NEOD001 in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction., PMID:26858336