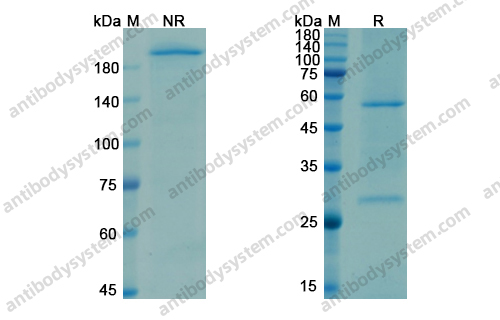
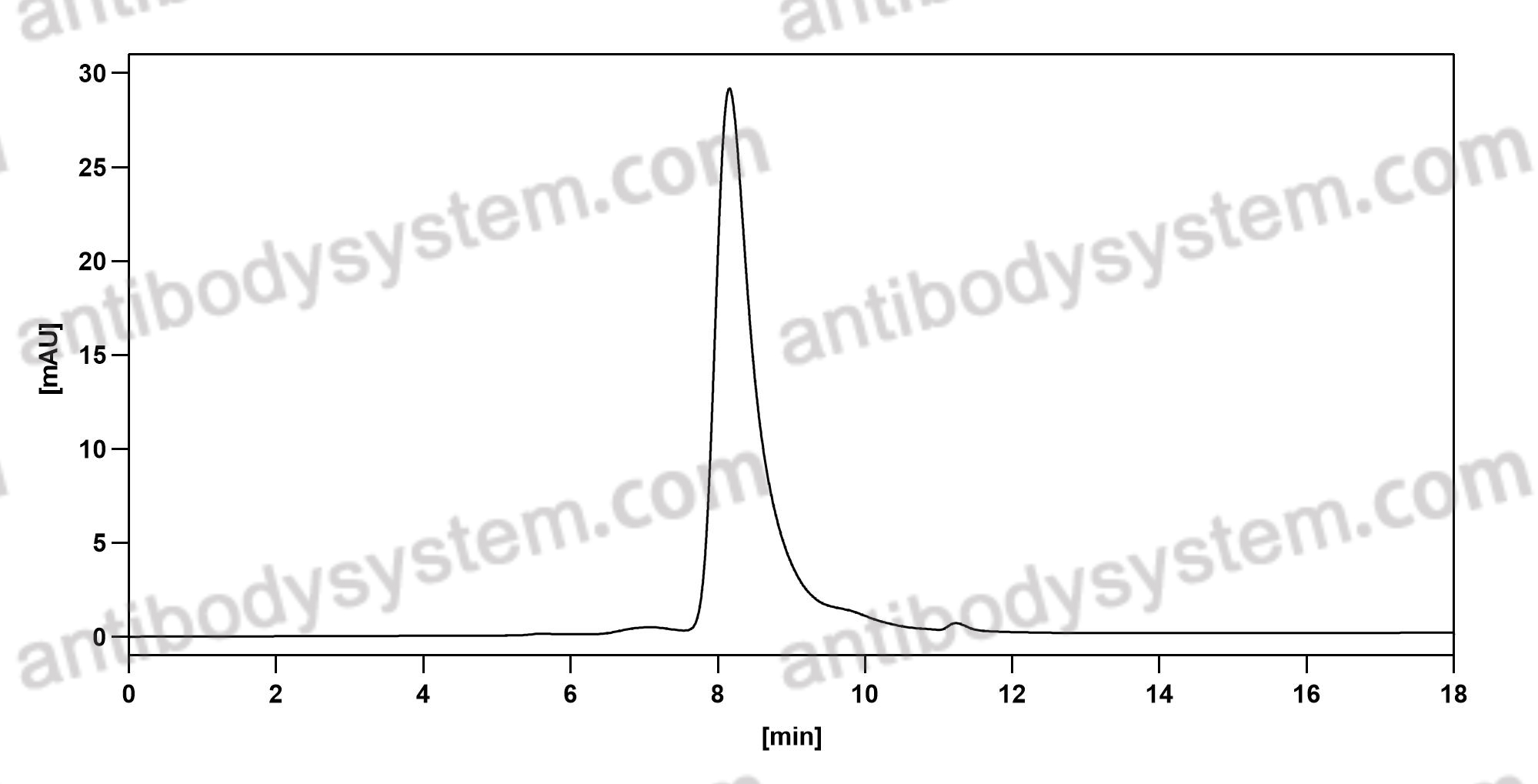
Catalog No.
DHJ39101
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
LRRN6A, LERN1, Leucine-rich repeat neuronal protein 1, Leucine-rich repeat and immunoglobulin domain-containing protein 1, LINGO1, Leucine-rich repeat neuronal protein 6A, Leucine-rich repeat and immunoglobulin-like domain-containing nogo receptor-interacting protein 1
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q96FE5
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
BIIB-033, CAS: 1422268-07-2
Clone ID
Opicinumab
Opicinumab: is it a potential treatment for multiple sclerosis?, PMID: 32793736
Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): a randomised, placebo-controlled, phase 2 trial, PMID: 31285147
Predictors of response to opicinumab in acute optic neuritis, PMID: 30349850
Anti lingo 1 (opicinumab) a new monoclonal antibody tested in relapsing remitting multiple sclerosis, PMID: 28885860
Remyelination in multiple sclerosis: from concept to clinical trials, PMID: 30865009
Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity, PMID: 29599194
Assessment of Opicinumab in Acute Optic Neuritis Using Multifocal Visual Evoked Potential, PMID: 30267385
Functional activity of anti-LINGO-1 antibody opicinumab requires target engagement at a secondary binding site, PMID: 31928294
Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis, PMID: 33258193
Initial Impairment and Recovery of Vision-Related Functioning in Participants With Acute Optic Neuritis From the RENEW Trial of Opicinumab, PMID: 30095536
Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial, PMID: 28229892
Approaches to Remyelination Therapies in Multiple Sclerosis, PMID: 31250211
Treatment of progressive multiple sclerosis: Challenges and promising perspectives, PMID: 29779852
Monoclonal Antibodies for Multiple Sclerosis: An Update, PMID: 30604390
Mechanisms and Medicines for Remyelination, PMID: 27860545
An unmet clinical need: roads to remyelination in MS, PMID: 33324887
Anti-LINGO-1 has no detectable immunomodulatory effects in preclinical and phase 1 studies, PMID: 29259995
Optic neuritis as a phase 2 paradigm for neuroprotection therapies of multiple sclerosis: update on current trials and perspectives, PMID: 27035900
Emerging myelin repair agents in preclinical and early clinical development for the treatment of multiple sclerosis, PMID: 32348161
Patterning Chronic Active Demyelination in Slowly Expanding/Evolving White Matter MS Lesions, PMID: 32819894
Remyelination therapies for multiple sclerosis: optimizing translation from animal models into clinical trials, PMID: 34126015
Interventions promoting remyelination in multiple sclerosis: a systematic review of clinical trials., PMID:40393003
RENEWED: A follow-up study of the opicinumab phase 2 RENEW study in participants with acute optic neuritis., PMID:39662163
Lesion-level correspondence and longitudinal properties of paramagnetic rim and slowly expanding lesions in multiple sclerosis., PMID:37036134
Novel Drugs in a Pipeline for Progressive Multiple Sclerosis., PMID:35743410
[Optic neuritis: aetiopathogenesis, diagnosis, prognosis and management]., PMID:35084734
Remyelination trial failures: Repercussions of ignoring neurorehabilitation and exercise in repair., PMID:35066276
Remyelination therapies for multiple sclerosis: optimizing translation from animal models into clinical trials., PMID:34126015
Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis., PMID:33258193
Patterning Chronic Active Demyelination in Slowly Expanding/Evolving White Matter MS Lesions., PMID:32819894
Opicinumab: is it a potential treatment for multiple sclerosis?, PMID:32793736
Emerging myelin repair agents in preclinical and early clinical development for the treatment of multiple sclerosis., PMID:32348161
Functional activity of anti-LINGO-1 antibody opicinumab requires target engagement at a secondary binding site., PMID:31928294
Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): a randomised, placebo-controlled, phase 2 trial., PMID:31285147
An unmet clinical need: roads to remyelination in MS., PMID:33324887
Approaches to Remyelination Therapies in Multiple Sclerosis., PMID:31250211
Remyelination in multiple sclerosis: from concept to clinical trials., PMID:30865009
Monoclonal Antibodies for Multiple Sclerosis: An Update., PMID:30604390
Predictors of response to opicinumab in acute optic neuritis., PMID:30349850
Assessment of Opicinumab in Acute Optic Neuritis Using Multifocal Visual Evoked Potential., PMID:30267385
Initial Impairment and Recovery of Vision-Related Functioning in Participants With Acute Optic Neuritis From the RENEW Trial of Opicinumab., PMID:30095536
Treatment of progressive multiple sclerosis: Challenges and promising perspectives., PMID:29779852
Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity., PMID:29599194
Anti-LINGO-1 has no detectable immunomodulatory effects in preclinical and phase 1 studies., PMID:29259995
Anti lingo 1 (opicinumab) a new monoclonal antibody tested in relapsing remitting multiple sclerosis., PMID:28885860
Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial., PMID:28229892
Mechanisms and Medicines for Remyelination., PMID:27860545
Optic neuritis as a phase 2 paradigm for neuroprotection therapies of multiple sclerosis: update on current trials and perspectives., PMID:27035900