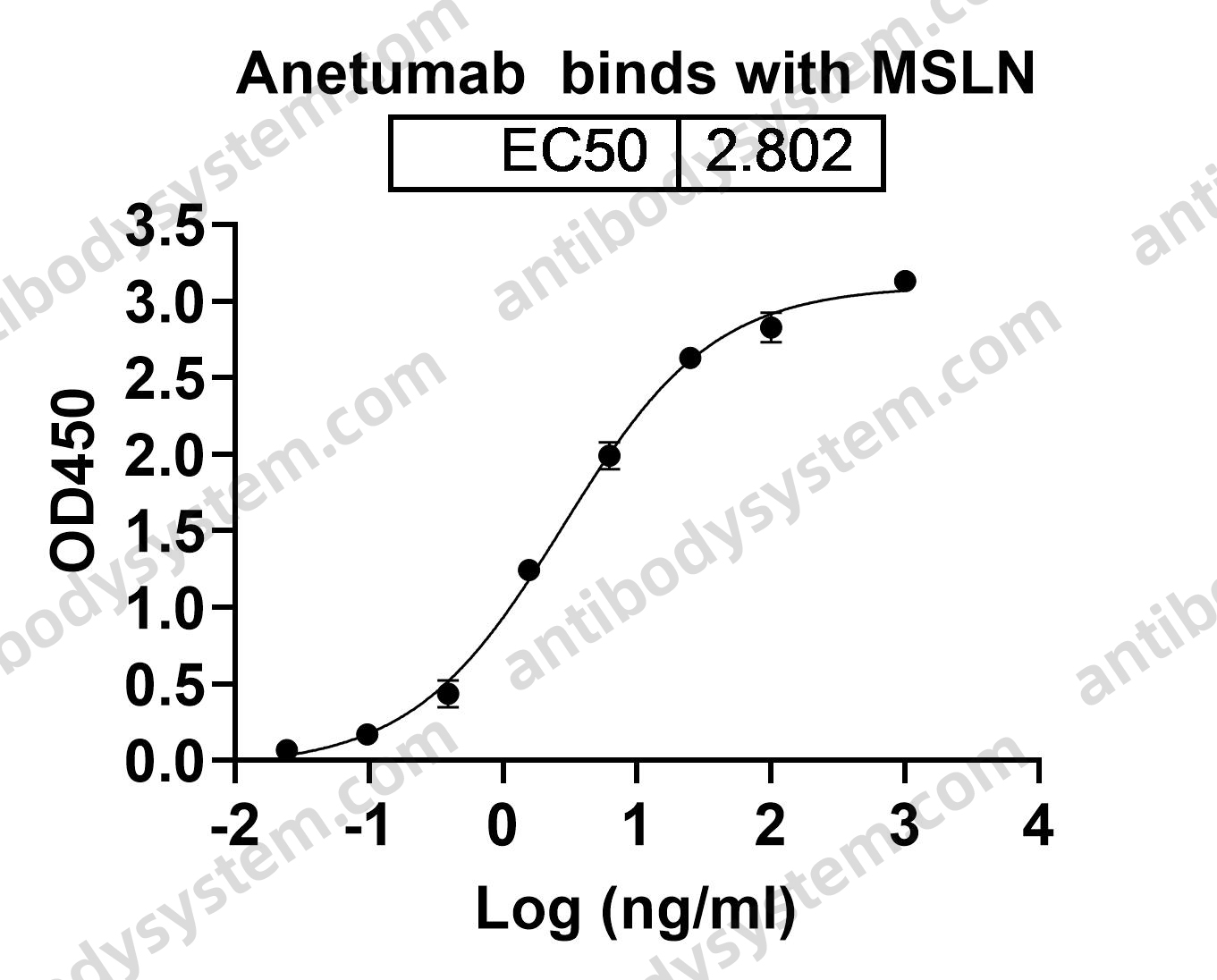
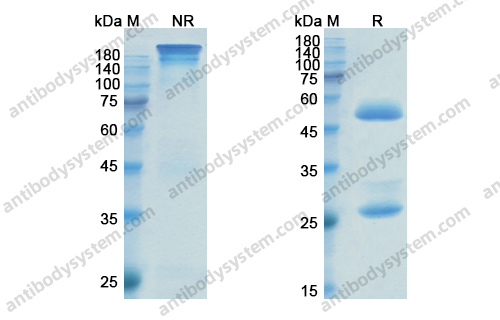
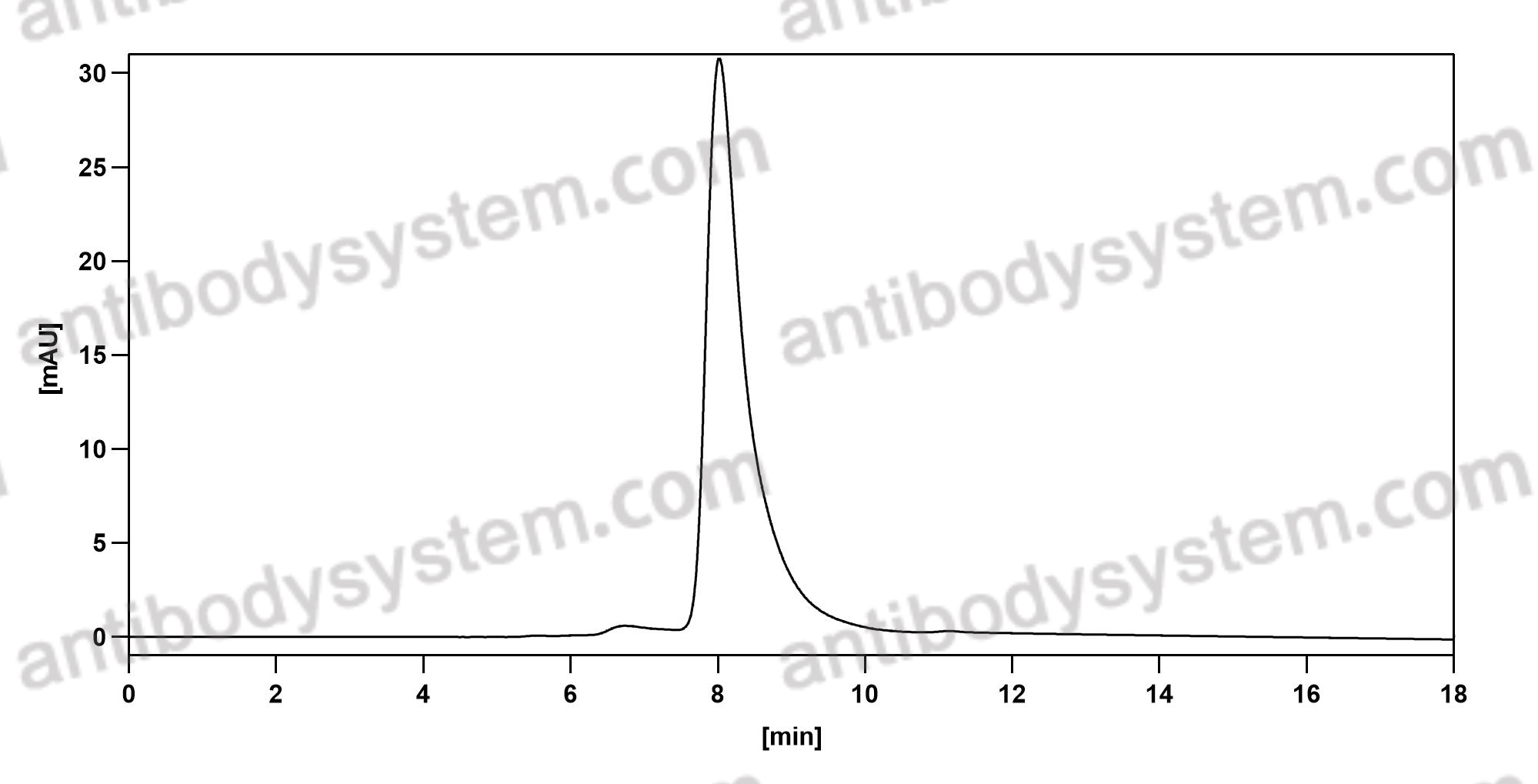
Catalog No.
DHG52001
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-lambda
Clonality
Monoclonal
Target
CAK1 antigen, Mesothelin, MSLN, Pre-pro-megakaryocyte-potentiating factor, MPF
Concentration
0.93 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q13421
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
BAY 94-9343, unconjugated: BAY 86-1903, BAY 2287409, Anetumab Ravtansine, CAS: 1954758-84-9
Clone ID
Anetumab
Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect, PMID: 24714131
First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors, PMID: 32213105
Anetumab ravtansine inhibits tumor growth and shows additive effect in combination with targeted agents and chemotherapy in mesothelin-expressing human ovarian cancer models, PMID: 30344925
Mesothelin Immunotherapy for Cancer: Ready for Prime Time?, PMID: 27863199
[Systemic Treatment of Malignant Pleural Mesothelioma], PMID: 29361614
Favorable therapeutic response after anti-Mesothelin antibody-drug conjugate treatment requires high expression of Mesothelin in tumor cells, PMID: 32815024
Mesothelin as a target for cervical cancer therapy, PMID: 30324544
Mesothelin expression in esophageal adenocarcinoma and squamous cell carcinoma and its possible impact on future treatment strategies, PMID: 32547645
Antibodies to watch in 2017, PMID: 27960628
Antibody-drug conjugates in gynecologic malignancies, PMID: 30929824
Targeting mesothelin in ovarian cancer, PMID: 30546824
Mesothelin is a novel cell surface disease marker and potential therapeutic target in acute myeloid leukemia, PMID: 33938941
Novel mesothelin antibody-drug conjugates: current evidence and future role in the treatment of ovarian cancer, PMID: 33356644
Novel Antibody Therapeutics Targeting Mesothelin In Solid Tumors, PMID: 27853672
Evaluating therapeutic plasma exchange and protease inhibitors as mechanisms to reduce soluble mesothelin., PMID:40240561
Evaluation of Innate Immune System, Body Habitus, and Sex on the Pharmacokinetics and Pharmacodynamics of Anetumab Ravtansine in Patients With Cancer., PMID:40051118
Development of a novel molecular probe for visualizing mesothelin on the tumor via positron emission tomography., PMID:39878895
Randomized Phase II Study of Bevacizumab with Weekly Anetumab Ravtansine or Weekly Paclitaxel in Platinum-Resistant/Refractory High-Grade Ovarian Cancer (NCI Trial)., PMID:39836408
Randomized trial of anetumab ravtansine and pembrolizumab compared to pembrolizumab for mesothelioma., PMID:39197359
Mesothelin promotes brain metastasis of non-small cell lung cancer by activating MET., PMID:38570866
Safety and activity of anti-mesothelin antibody-drug conjugate anetumab ravtansine in combination with pegylated-liposomal doxorubicin in platinum-resistant ovarian cancer: multicenter, phase Ib dose escalation and expansion study., PMID:36564099
Anetumab ravtansine versus vinorelbine in patients with relapsed, mesothelin-positive malignant pleural mesothelioma (ARCS-M): a randomised, open-label phase 2 trial., PMID:35358455
Salvage Therapy for Relapsed Malignant Pleural Mesothelioma: A Systematic Review and Network Meta-Analysis., PMID:35008346
Exploiting mesothelin in thymic carcinoma as a drug delivery target for anetumab ravtansine., PMID:34876673
In Vitro and In Vivo Comparison of 3,2-HOPO Versus Deferoxamine-Based Chelation of Zirconium-89 to the Antimesothelin Antibody Anetumab., PMID:34014767
Mesothelin is a novel cell surface disease marker and potential therapeutic target in acute myeloid leukemia., PMID:33938941
Novel mesothelin antibody-drug conjugates: current evidence and future role in the treatment of ovarian cancer., PMID:33356644
Favorable therapeutic response after anti-Mesothelin antibody-drug conjugate treatment requires high expression of Mesothelin in tumor cells., PMID:32815024
Mesothelin expression in esophageal adenocarcinoma and squamous cell carcinoma and its possible impact on future treatment strategies., PMID:32547645
First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors., PMID:32213105
Antibody-drug conjugates in gynecologic malignancies., PMID:30929824
Targeting mesothelin in ovarian cancer., PMID:30546824
Anetumab ravtansine inhibits tumor growth and shows additive effect in combination with targeted agents and chemotherapy in mesothelin-expressing human ovarian cancer models., PMID:30344925
Mesothelin as a target for cervical cancer therapy., PMID:30324544
[Systemic Treatment of Malignant Pleural Mesothelioma]., PMID:29361614
Antibodies to watch in 2017., PMID:27960628
Mesothelin Immunotherapy for Cancer: Ready for Prime Time?, PMID:27863199
Novel Antibody Therapeutics Targeting Mesothelin In Solid Tumors., PMID:27853672
Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect., PMID:24714131