Catalog No.
DHF14604
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-lambda
Clonality
Monoclonal
Target
IgG Fc fragment receptor transporter alpha chain, IgG receptor FcRn large subunit p51, FcRn, FCGRT, FCRN, Neonatal Fc receptor
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
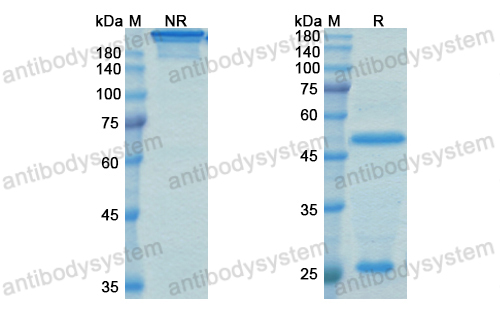
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P55899
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
M-281, M281, CAS: 2211985-36-1
Clone ID
Nipocalimab
Medical therapy to attenuate fetal anaemia in severe maternal red cell alloimmunisation, PMID: 32794242
The importance of FcRn in neuro-immunotherapies: From IgG catabolism, FCGRT gene polymorphisms, IVIg dosing and efficiency to specific FcRn inhibitors, PMID: 33717213
Psychometric analysis of the Neuro-QoL Fatigue in generalized Myasthenia Gravis (gMG) using data from a phase 3 trial., PMID:40515798
Pharmacokinetics, Pharmacodynamics, and Safety of Nipocalimab in Healthy Chinese Volunteers: A Single-Dose, Phase I Study., PMID:40389701
Efficacy and safety of FcRn inhibitors in patients with Myasthenia gravis: An updated systematic review and meta‑analysis., PMID:40288289
The Utility of a Critical Antibody Titer in Anti-K Alloimmunized Pregnancies: A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy., PMID:40253764
Endocytotic Albumin Uptake Pathways in Human Adipose Stem Cells and Connection to Intracellular Calcium Oscillations and the Neonatal Fc receptor., PMID:40239638
A randomized, open-label study on the effect of nipocalimab on vaccine responses in healthy participants., PMID:40232701
Considerations and approaches for early onset fetal anemia due to red cell alloimmunization., PMID:40179608
Plain Language Summary of Publication: Design of the Phase 3 AZALEA Trial of Nipocalimab in Severe Hemolytic Disease of the Fetus and Newborn., PMID:40049596
Nipocalimab, an immunoselective FcRn blocker that lowers IgG and has unique molecular properties., PMID:39936406
FcRn inhibitors in the context of myasthenia gravis., PMID:39870595
Safety and efficacy of nipocalimab in adults with generalised myasthenia gravis (Vivacity-MG3): a phase 3, randomised, double-blind, placebo-controlled study., PMID:39862879
What Is in the Neuromuscular Junction Literature?, PMID:39590927
Initiation response, maximized therapeutic efficacy, and post-treatment effects of biological targeted therapies in myasthenia gravis: a systematic review and network meta-analysis., PMID:39529623
Antigen-specific immunotherapy for platelet alloimmune disorders., PMID:39520801
[Neonatal Fc receptor : role and implications in maternal fetal medicine]., PMID:39429174
Autoimmune Hemolytic Anemias: Challenges in Diagnosis and Therapy., PMID:39371250
Current state and potential applications of neonatal Fc receptor (FcRn) inhibitors in hematologic conditions., PMID:39324647
Design of a Phase 3, Global, Multicenter, Randomized, Placebo-Controlled, Double-Blind Study of Nipocalimab in Pregnancies at Risk for Severe Hemolytic Disease of the Fetus and Newborn., PMID:39197469
Management of Red Cell Alloimmunization in Pregnancy., PMID:39146538
Nipocalimab in Early-Onset Severe Hemolytic Disease of the Fetus and Newborn., PMID:39115062
Pharmacokinetics and Pharmacodynamics of Nipocalimab, a Neonatal Fc Receptor Blocker, in Healthy Japanese Volunteers., PMID:39073504
Nipocalimab, an anti-FcRn monoclonal antibody, in participants with moderate to severe active rheumatoid arthritis and inadequate response or intolerance to anti-TNF therapy: results from the phase 2a IRIS-RA study., PMID:38942592
FcRn Inhibitor Therapies in Neurologic Diseases., PMID:38724842
Maternal red blood cell alloimmunization prevalence in the United States., PMID:38662646
Chronic Inflammatory Demyelinating Polyradiculoneuropathy: Current Therapeutic Approaches and Future Outlooks., PMID:38435981
The efficacy and safety of FcRn inhibitors in patients with myasthenia gravis: a systematic review and meta-analysis., PMID:38431900
Pharmacokinetics and pharmacodynamics across infusion rates of intravenously administered nipocalimab: results of a phase 1, placebo-controlled study., PMID:38362023
The Efficacy and Safety of Different Targeted Drugs for the Treatment of Generalized Myasthenia Gravis: A Systematic Review and Bayesian Network Meta-analysis., PMID:38300476
Safety and Efficacy of Nipocalimab in Patients With Generalized Myasthenia Gravis: Results From the Randomized Phase 2 Vivacity-MG Study., PMID:38165333
Efficacy and safety of the innovative monoclonal antibodies in adults with generalized myasthenia gravis: a Bayesian network analysis., PMID:38022544
Antibody based therapeutics for autoimmune hemolytic anemia., PMID:37874225
Pregnant Women at Low Risk of Having a Child with Fetal and Neonatal Alloimmune Thrombocytopenia Do Not Require Treatment with Intravenous Immunoglobulin., PMID:37685558
FcRN receptor antagonists in the management of myasthenia gravis., PMID:37602255
Janssen's nipocalimab tackles hemolytic disease of the fetus and newborn., PMID:36859603
FcRn inhibitors: a novel option for the treatment of myasthenia gravis., PMID:36751773
ABO Incompatibility between the Mother and Fetus Does Not Protect against Anti-Human Platelet Antigen-1a Immunization by Pregnancy., PMID:36431288
New Therapies for the Treatment of Warm Autoimmune Hemolytic Anemia., PMID:36182620
Pharmacotherapy of Generalized Myasthenia Gravis with Special Emphasis on Newer Biologicals., PMID:35639288
The importance of FcRn in neuro-immunotherapies: From IgG catabolism, FCGRT gene polymorphisms, IVIg dosing and efficiency to specific FcRn inhibitors., PMID:33717213
Medical therapy to attenuate fetal anaemia in severe maternal red cell alloimmunisation., PMID:32794242