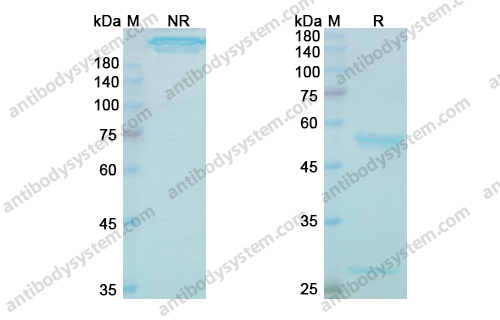
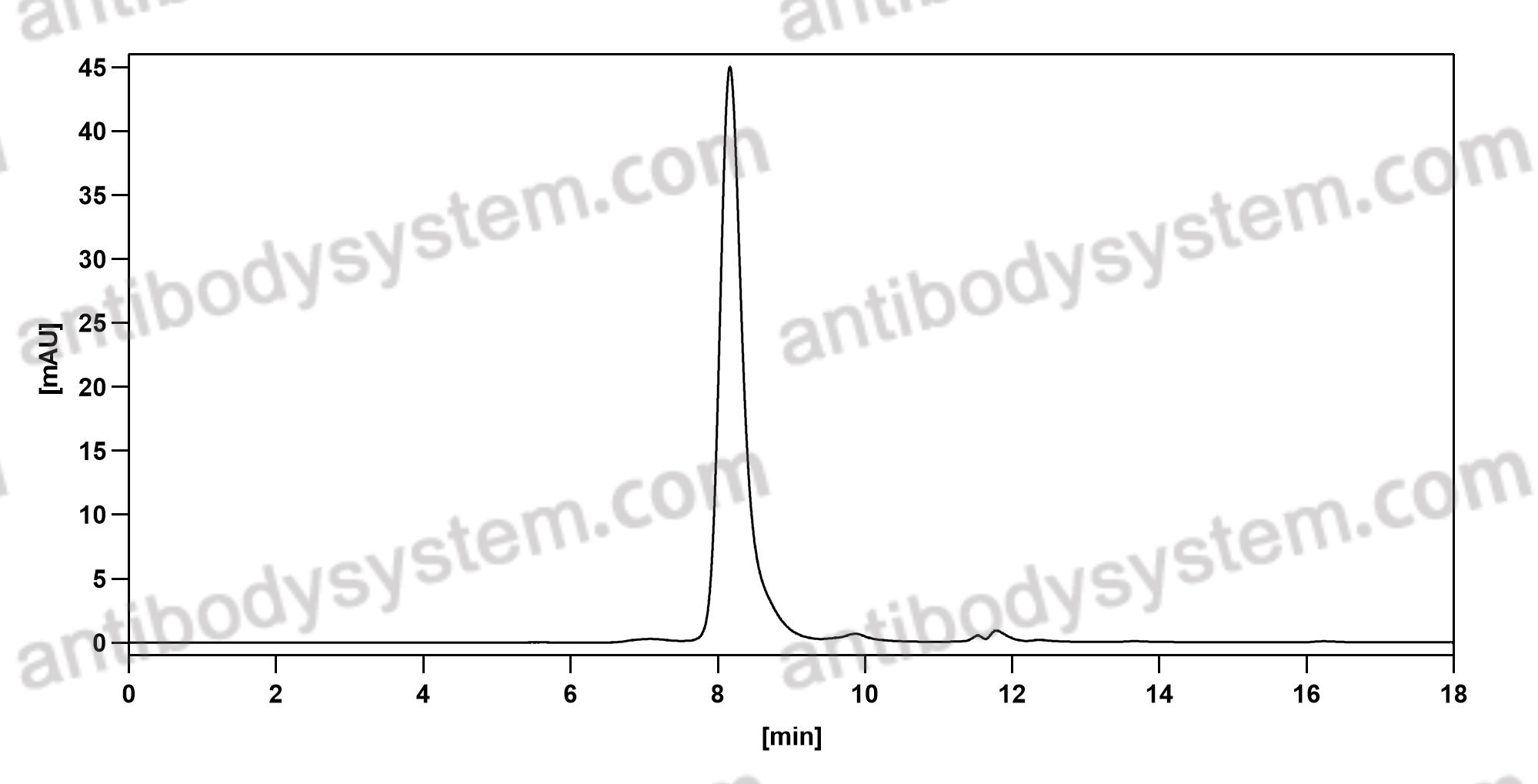
Catalog No.
DHF14603
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-lambda
Clonality
Monoclonal
Target
IgG Fc fragment receptor transporter alpha chain, IgG receptor FcRn large subunit p51, FcRn, FCGRT, FCRN, Neonatal Fc receptor
Concentration
1.29 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P55899
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
HL161, CAS: 2187430-05-1
Clone ID
Batoclimab
Comparative efficacy and acceptability of novel biologics in the treatment of myasthenia gravis: systematic review and network meta-analysis of randomized trials., PMID:40346603
Impact of Batoclimab Treatment on LDL-C with and Without Coadministration of Atorvastatin: Results from a Phase I Randomized Study in Healthy Participants., PMID:40287571
Targeted immunotherapies for Graves' thyroidal & orbital diseases., PMID:40145088
FcRn inhibitors in the context of myasthenia gravis., PMID:39870595
Initiation response, maximized therapeutic efficacy, and post-treatment effects of biological targeted therapies in myasthenia gravis: a systematic review and network meta-analysis., PMID:39529623
Efficacy and safety of immunosuppressants and monoclonal antibodies in adults with myasthenia gravis: a systematic review and network meta-analysis., PMID:39434135
FcRn Inhibitor Therapies in Neurologic Diseases., PMID:38724842
Batoclimab vs Placebo for Generalized Myasthenia Gravis: A Randomized Clinical Trial., PMID:38436998
Chronic Inflammatory Demyelinating Polyradiculoneuropathy: Current Therapeutic Approaches and Future Outlooks., PMID:38435981
The efficacy and safety of FcRn inhibitors in patients with myasthenia gravis: a systematic review and meta-analysis., PMID:38431900
The Efficacy and Safety of Different Targeted Drugs for the Treatment of Generalized Myasthenia Gravis: A Systematic Review and Bayesian Network Meta-analysis., PMID:38300476
Batoclimab as induction and maintenance therapy in patients with myasthenia gravis: rationale and study design of a phase 3 clinical trial., PMID:38268752
The position of monoclonal antibodies and small molecules in the treatment of thyroid orbitopathy., PMID:40091323
Subcutaneous batoclimab in generalized myasthenia gravis: Results from a Phase 2a trial with an open-label extension., PMID:38062618
Efficacy and safety of the innovative monoclonal antibodies in adults with generalized myasthenia gravis: a Bayesian network analysis., PMID:38022544
FcRN receptor antagonists in the management of myasthenia gravis., PMID:37602255
Proof-of-concept and Randomized, Placebo-controlled Trials of an FcRn Inhibitor, Batoclimab, for Thyroid Eye Disease., PMID:37390454
FcRn inhibitors: a novel option for the treatment of myasthenia gravis., PMID:36751773
Batoclimab as an add-on therapy in neuromyelitis optica spectrum disorder patients with acute attacks., PMID:36087008
Pharmacotherapy of Generalized Myasthenia Gravis with Special Emphasis on Newer Biologicals., PMID:35639288
Therapeutic Effects of Batoclimab in Chinese Patients with Generalized Myasthenia Gravis: A Double-Blinded, Randomized, Placebo-Controlled Phase II Study., PMID:35412216