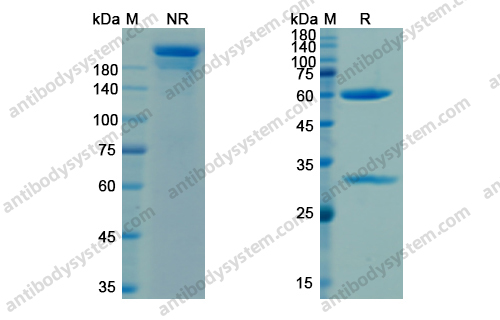
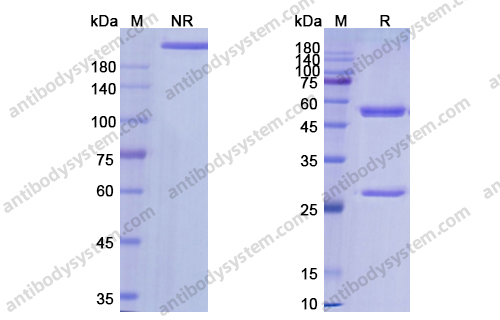
Catalog No.
DHC34203
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Tyrosine-protein kinase Met, Hepatocyte growth factor receptor, Proto-oncogene c-Met, Scatter factor receptor, HGF receptor, SF receptor, HGF/SF receptor, MET
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P08581
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
ABBV-399, ABT-700-vcMMAE, ABT-700, hz224G11, CAS: 1781223-80-0
Clone ID
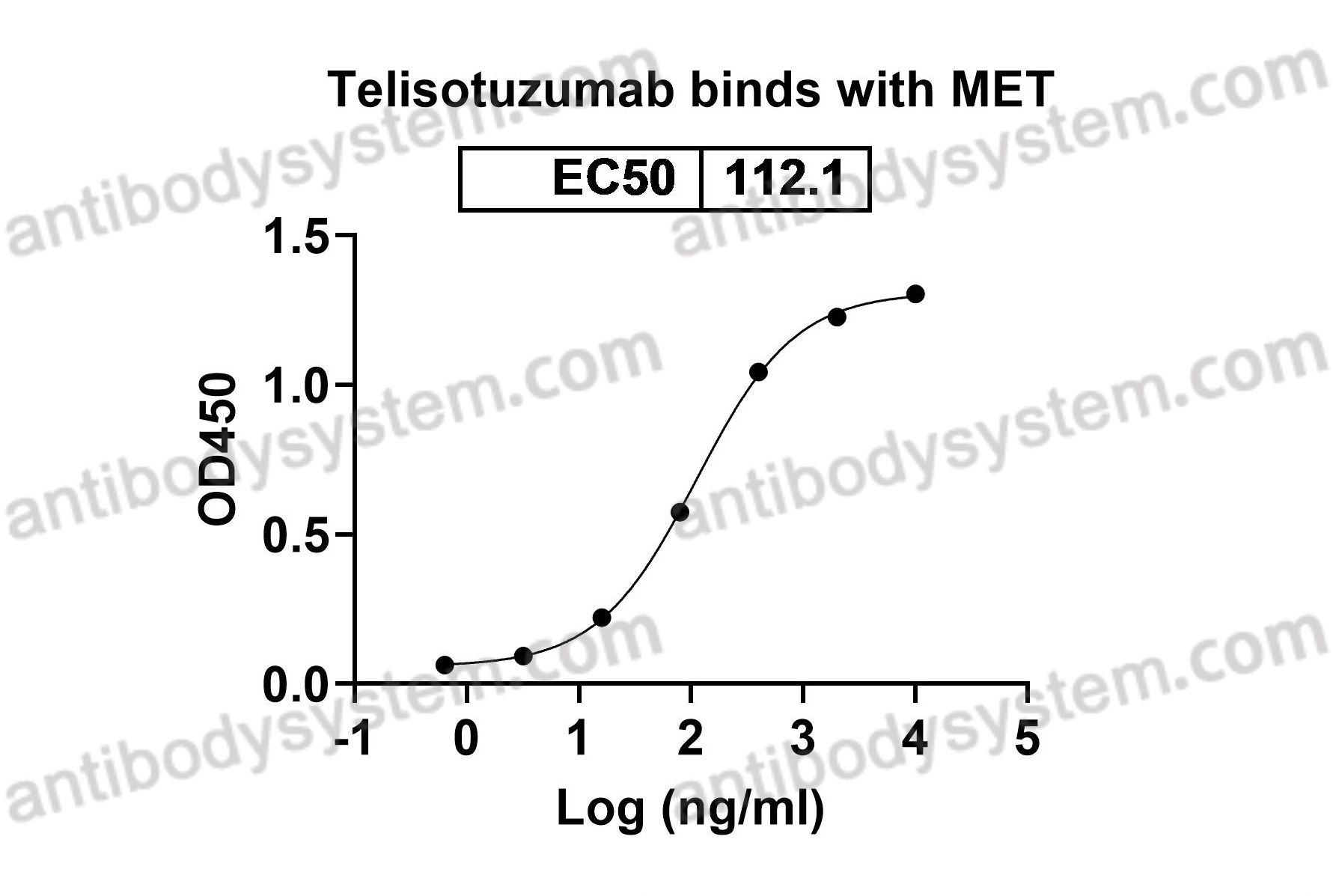
Telisotuzumab
First-in-Human Phase I, Dose-Escalation and -Expansion Study of Telisotuzumab Vedotin, an Antibody-Drug Conjugate Targeting c-Met, in Patients With Advanced Solid Tumors, PMID: 30285518
Phase I Dose-Escalation and -Expansion Study of Telisotuzumab (ABT-700), an Anti-c-Met Antibody, in Patients with Advanced Solid Tumors, PMID: 32127466
A Phase II Study of Telisotuzumab Vedotin in Patients With c-MET-positive Stage IV or Recurrent Squamous Cell Lung Cancer (LUNG-MAP Sub-study S1400K, NCT03574753), PMID: 33221175
Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): a biomarker-driven master protocol, PMID: 33125909
Phase 1 study of telisotuzumab vedotin in Japanese patients with advanced solid tumors, PMID: 33675179
Biotherapeutic Antibodies for the Treatment of Head and Neck Cancer: Current Approaches and Future Considerations of Photothermal Therapies, PMID: 33324546
Phase 1 Study of 2- or 3-Week Dosing of Telisotuzumab Vedotin, an Antibody-Drug Conjugate Targeting c-Met, Monotherapy in Patients With Advanced Non-Small Cell Lung Carcinoma, PMID: 34426443
Targeted Therapy Approaches for MET Abnormalities in Non-Small Cell Lung Cancer, PMID: 33638808
ABBV-399, a c-Met Antibody-Drug Conjugate that Targets Both MET-Amplified and c-Met-Overexpressing Tumors, Irrespective of MET Pathway Dependence, PMID: 27573171
Efficacy and Safety of Antibody-Drug Conjugates for Lung Cancer Therapy: A Systematic Review of Randomized and Non-Randomized Clinical Trials., PMID:40430899
[Diffuse interstitial lung disease induced by antibody-drug conjugates]., PMID:40263022
Recent advances in therapeutic strategies for non-small cell lung cancer., PMID:40140911
Treatment of c-MET Antibody-Drug Conjugate Asymptomatic Pneumonitis with a Novel Steroid Regimen in Non-Small Cell Lung Cancer: A Case Report., PMID:40066205
Telisotuzumab vedotin and osimertinib: the METamorphosis of epidermal growth factor receptor-mutant lung cancer?, PMID:39855428
Results from a phase Ib study of telisotuzumab vedotin in combination with osimertinib in patients with c-Met protein-overexpressing, EGFR-mutated locally advanced/metastatic non-small-cell lung cancer (NSCLC) after progression on prior osimertinib., PMID:39805351
Antibodies to watch in 2025., PMID:39711140
Anti-MET Antibody Therapies in Non-Small-Cell Lung Cancer: Current Progress and Future Directions., PMID:39449330
Telisotuzumab Vedotin Monotherapy in Patients With Previously Treated c-Met Protein-Overexpressing Advanced Nonsquamous EGFR-Wildtype Non-Small Cell Lung Cancer in the Phase II LUMINOSITY Trial., PMID:38843488
Antibody-drug conjugates in lung and breast cancer: current evidence and future directions-a position statement from the ETOP IBCSG Partners Foundation., PMID:38648979
Telisotuzumab vedotin with erlotinib in the treatment of non-small cell lung cancer: a well MET combination?, PMID:37691875
Antibody-drug conjugates in lung cancer: dawn of a new era?, PMID:36631624
Phase Ib Study of Telisotuzumab Vedotin in Combination With Erlotinib in Patients With c-Met Protein-Expressing Non-Small-Cell Lung Cancer., PMID:36288547
Case report: Salvage capmatinib therapy in KIF5B-MET fusion-positive lung adenocarcinoma with resistance to telisotuzumab vedotin., PMID:36033470
A Phase 1b Study of Telisotuzumab Vedotin in Combination With Nivolumab in Patients With NSCLC., PMID:35005654
Antibody-drug conjugates: A promising novel therapeutic approach in lung cancer., PMID:34942494
Phase I Study of 2- or 3-Week Dosing of Telisotuzumab Vedotin, an Antibody-Drug Conjugate Targeting c-Met, Monotherapy in Patients with Advanced Non-Small Cell Lung Carcinoma., PMID:34426443
Phase 1 study of telisotuzumab vedotin in Japanese patients with advanced solid tumors., PMID:33675179
Targeted Therapy Approaches for MET Abnormalities in Non-Small Cell Lung Cancer., PMID:33638808
Biotherapeutic Antibodies for the Treatment of Head and Neck Cancer: Current Approaches and Future Considerations of Photothermal Therapies., PMID:33324546
A Phase II Study of Telisotuzumab Vedotin in Patients With c-MET-positive Stage IV or Recurrent Squamous Cell Lung Cancer (LUNG-MAP Sub-study S1400K, NCT03574753)., PMID:33221175
Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): a biomarker-driven master protocol., PMID:33125909
Phase I Dose-Escalation and -Expansion Study of Telisotuzumab (ABT-700), an Anti-c-Met Antibody, in Patients with Advanced Solid Tumors., PMID:32127466
First-in-Human Phase I, Dose-Escalation and -Expansion Study of Telisotuzumab Vedotin, an Antibody-Drug Conjugate Targeting c-Met, in Patients With Advanced Solid Tumors., PMID:30285518
ABBV-399, a c-Met Antibody-Drug Conjugate that Targets Both MET-Amplified and c-Met-Overexpressing Tumors, Irrespective of MET Pathway Dependence., PMID:27573171