Catalog No.
DHA30603
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
GDF-8, GDF8, MSTN, Myostatin, Growth/differentiation factor 8
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
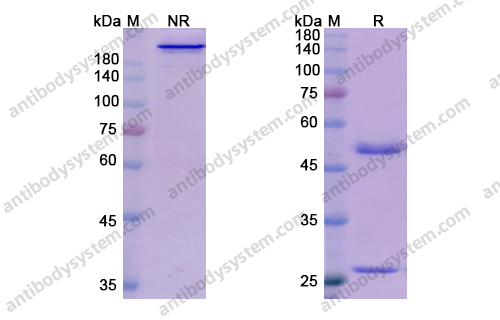
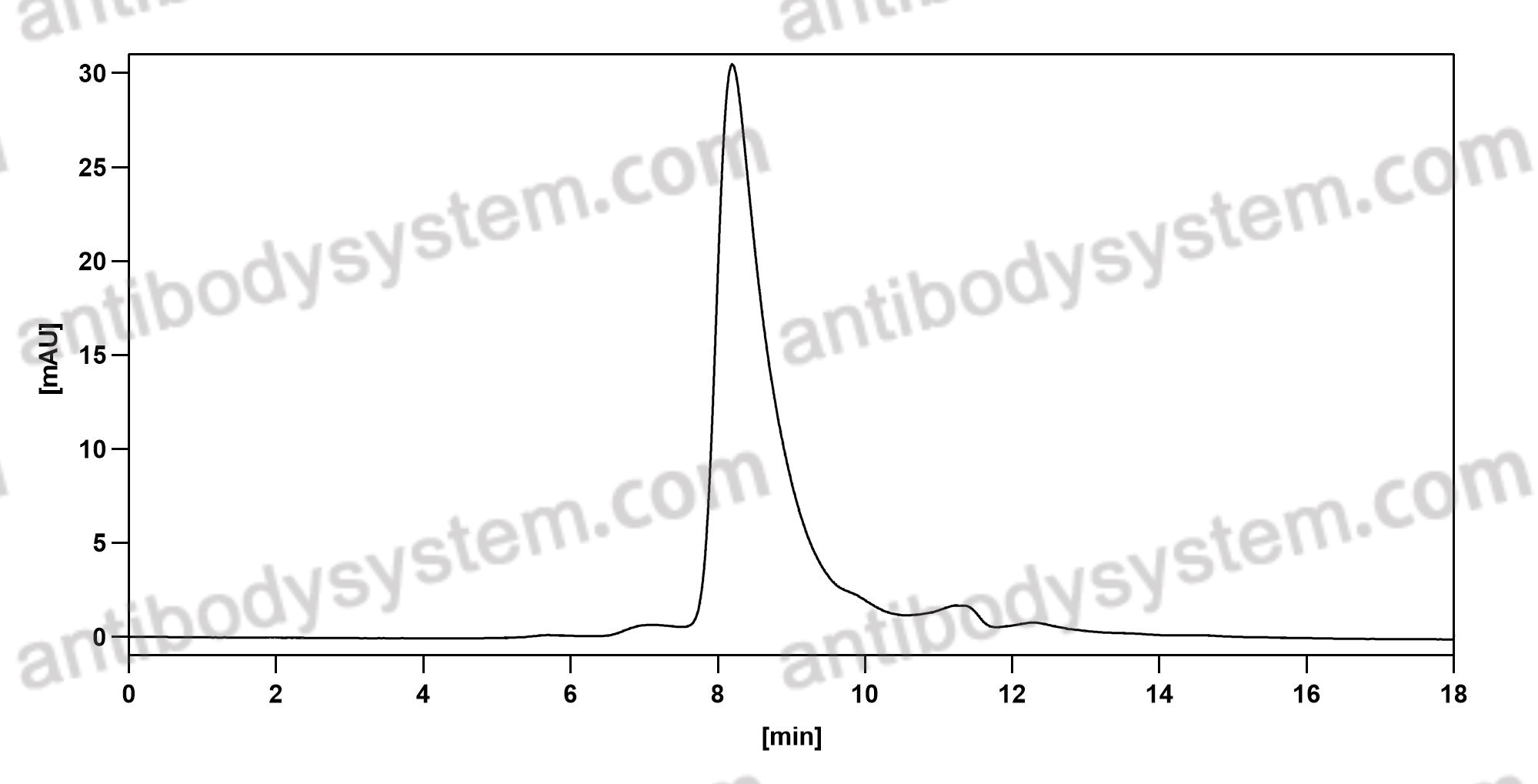
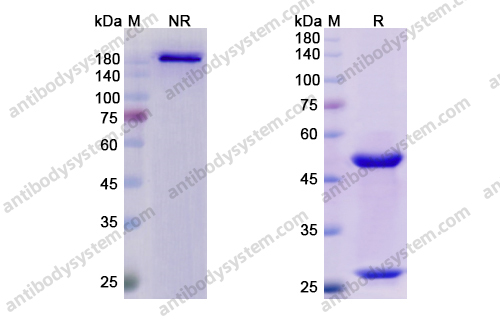
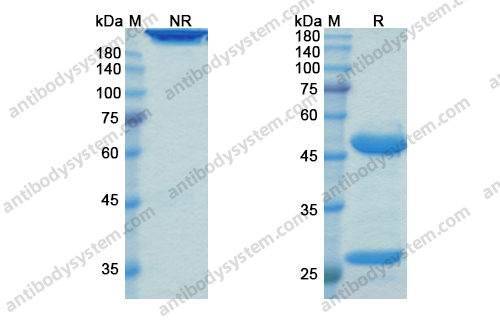
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
O14793
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
PF-06252616, CAS: 1629605-31-7
Clone ID
Domagrozumab
Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy, PMID: 32522498
Comparing Model Performance in Characterizing the PK/PD of the Anti-Myostatin Antibody Domagrozumab, PMID: 31550073
Detection of the myostatin-neutralizing antibody Domagrozumab in serum by means of Western blotting and LC-HRMS, PMID: 31692253
Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Domagrozumab (PF-06252616), an Antimyostatin Monoclonal Antibody, in Healthy Subjects, PMID: 28881472
A mouse anti-myostatin antibody increases muscle mass and improves muscle strength and contractility in the mdx mouse model of Duchenne muscular dystrophy and its humanized equivalent, domagrozumab (PF-06252616), increases muscle volume in cynomolgus monkeys, PMID: 29121992
Application of Quantitative Pharmacology Approaches in Bridging Pharmacokinetics and Pharmacodynamics of Domagrozumab From Adult Healthy Subjects to Pediatric Patients With Duchenne Muscular Disease, PMID: 29023829
Corrigendum to "Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy" [Neuromuscular Disorders, Vol. 30 (6) 2020, 492-502], PMID: 33451933
A phase Ib/IIa, open-label, multiple ascending-dose trial of domagrozumab in fukutin-related protein limb-girdle muscular dystrophy, PMID: 33961310
Quantitative muscle MRI biomarkers in Duchenne muscular dystrophy: cross-sectional correlations with age and functional tests, PMID: 34155911
A Generic Detection Method for the Doping Control Analysis of Fc-Fusion Proteins and Monoclonal Antibodies in Equine Plasma., PMID:40033065
Relationship Between Quantitative Magnetic Resonance Imaging Measures and Functional Changes in Patients With Duchenne Muscular Dystrophy., PMID:39713935
Simultaneous detection of myostatin-targeting monoclonal antibodies in dried blood spots and plasma using liquid chromatography-tandem mass spectrometry with field asymmetric ion mobility spectrometry., PMID:39405785
Dual-energy X-ray absorptiometry measures of lean body mass as a biomarker for progression in boys with Duchenne muscular dystrophy., PMID:36335191
Population PK and PD Analysis of Domagrozumab in Pediatric Patients with Duchenne Muscular Dystrophy., PMID:36104012
Novel approaches to analysis of the North Star Ambulatory Assessment (NSAA) in Duchenne muscular dystrophy (DMD): Observations from a phase 2 trial., PMID:35998119
Quantitative magnetic resonance imaging measures as biomarkers of disease progression in boys with Duchenne muscular dystrophy: a phase 2 trial of domagrozumab., PMID:35396602
Safety and disease monitoring biomarkers in Duchenne muscular dystrophy: results from a Phase II trial., PMID:34533053
Quantitative muscle MRI biomarkers in Duchenne muscular dystrophy: cross-sectional correlations with age and functional tests., PMID:34155911
A phase Ib/IIa, open-label, multiple ascending-dose trial of domagrozumab in fukutin-related protein limb-girdle muscular dystrophy., PMID:33961310
Corrigendum to "Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy" [Neuromuscular Disorders, Vol. 30 (6) 2020, 492-502]., PMID:33451933
Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy., PMID:32522498
Detection of the myostatin-neutralizing antibody Domagrozumab in serum by means of Western blotting and LC-HRMS., PMID:31692253
Comparing Model Performance in Characterizing the PK/PD of the Anti-Myostatin Antibody Domagrozumab., PMID:31550073
A mouse anti-myostatin antibody increases muscle mass and improves muscle strength and contractility in the mdx mouse model of Duchenne muscular dystrophy and its humanized equivalent, domagrozumab (PF-06252616), increases muscle volume in cynomolgus monkeys., PMID:29121992
Application of Quantitative Pharmacology Approaches in Bridging Pharmacokinetics and Pharmacodynamics of Domagrozumab From Adult Healthy Subjects to Pediatric Patients With Duchenne Muscular Disease., PMID:29023829
Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Domagrozumab (PF-06252616), an Antimyostatin Monoclonal Antibody, in Healthy Subjects., PMID:28881472