Catalog No.
DHJ24007
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG2-kappa
Clonality
Monoclonal
Target
Proprotein convertase subtilisin/kexin type 9, Subtilisin/kexin-like protease PC9, NARC-1, PC9, Proprotein convertase 9, Neural apoptosis-regulated convertase 1, NARC1, PCSK9
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
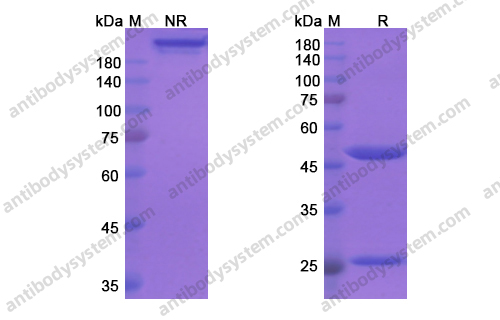
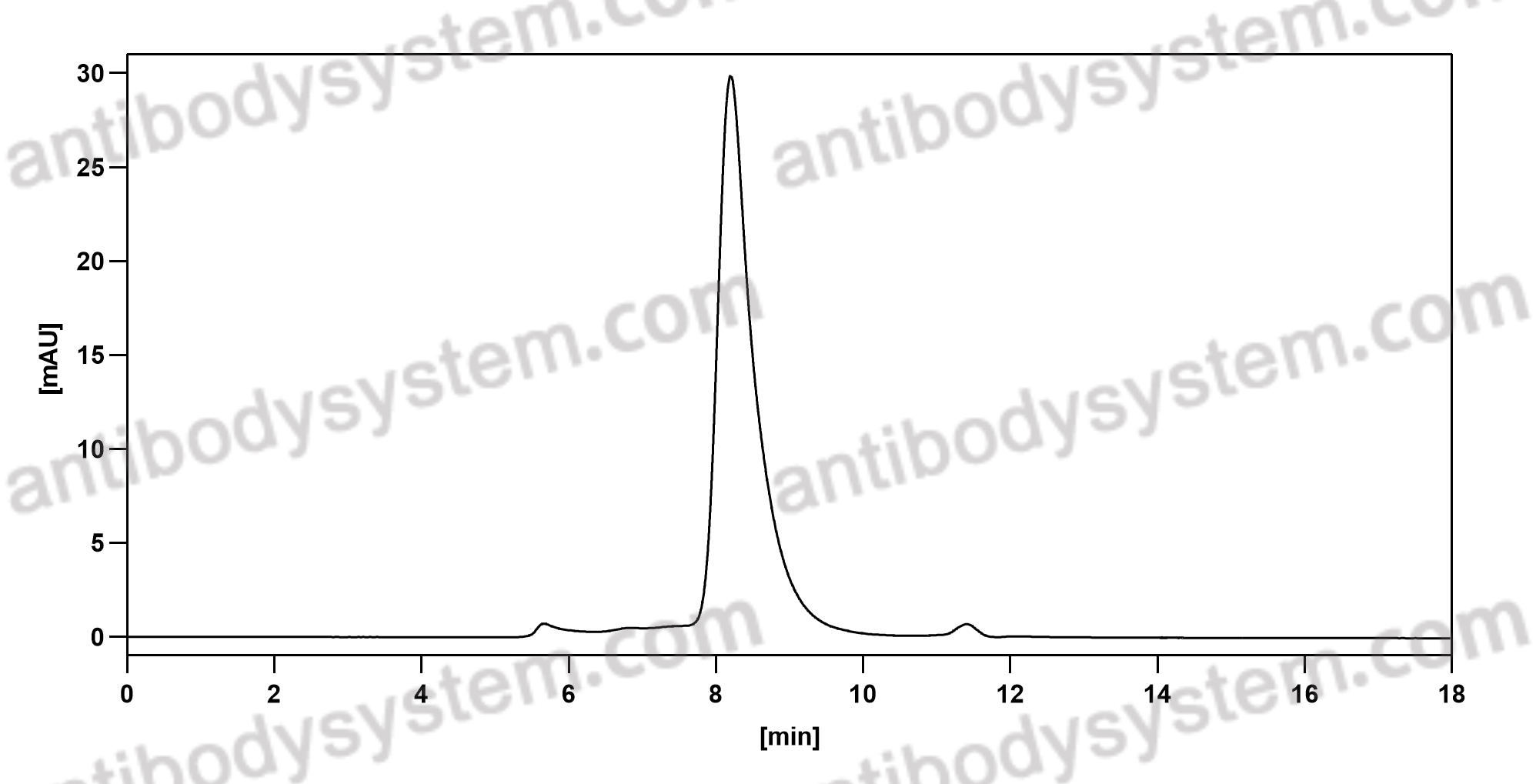
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q8NBP7
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
IBI-306, CAS: 2225109-03-3
Clone ID
Tafolecimab
Efficacy and safety of tafolecimab a new PCSK9 inhibitor in patients with hyperlipidemia: a systematic review and meta-analysis of randomized controlled trials., PMID:40478366
Cost-Effectiveness and Price Threshold Analysis of Tafolecimab in Chinese Patients with Elevated LDL Cholesterol Despite Statin Therapy., PMID:40175801
Tafolecimab, a Third Monoclonal Antibody for PCSK9 as the Novel Lipid-Lowering Drug Around the World: A Narrative Review., PMID:40167967
Comprehensive Assessment of PCSK9 Inhibitors for Lipid Management: Scientific Guidance Based on Drug Selection Recommendations for Chinese Medical Institutions., PMID:39741915
The efficacy and safety of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors combined with statins in patients with hypercholesterolemia: a network meta-analysis., PMID:39386388
The Efficacy of Tafolecimab in Chinese Patients with Hypercholesterolemia: A Systematic Review and Meta-analysis., PMID:38913274
Hypercholesterolemia: a literature review on management using tafolecimab: a novel member of PCSK9 monoclonal antibodies., PMID:38694324
Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): from bench to bedside., PMID:38185721
Antibodies to watch in 2024., PMID:38178784
Pharmacokinetic/LDL-C and exposure-response analysis of tafolecimab in Chinese hypercholesterolemia patients: Results from phase I, II, and III studies., PMID:37877498
Tafolecimab: First Approval., PMID:37847461
Tafolecimab in Chinese patients with non-familial hypercholesterolemia (CREDIT-1): a 48-week randomized, double-blind, placebo-controlled phase 3 trial., PMID:37808342
Tafolecimab in Chinese Patients With Hypercholesterolemia (CREDIT-4): A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial., PMID:37614541
Tafolecimab, A Novel Member of PCSK9 Monoclonal Antibodies, Is Worth Expecting in a Chinese Population., PMID:37614538
Efficacy and safety of tafolecimab in Chinese patients with heterozygous familial hypercholesterolemia: a randomized, double-blind, placebo-controlled phase 3 trial (CREDIT-2)., PMID:36855099
A Potential Long-Acting LDL-Cholesterol-Lowering PCSK9 Monoclonal Antibody: Randomized, Placebo-Controlled Phase 1 Studies., PMID:36341216