Catalog No.
DHC12502
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
AICD-57, A4, Beta-CTF, Protease nexin-II, Gamma-CTF(50), ABPP, S-APP-beta, APP, Abeta40, Abeta42, AID(59), Amyloid intracellular domain 59, AID(57), S-APP-alpha, Gamma-CTF(59), Beta-secretase C-terminal fragment, Amyloid-beta precursor protein, Amyloid precursor protein, Beta-APP42, PreA4, Alzheimer disease amyloid protein, APPI, PN-II, AICD-59, Alpha-secretase C-terminal fragment, Amyloid-beta A4 protein, Amyloid intracellular domain 57, CVAP, Amyloid intracellular domain 50, Beta-APP40, AD1, Cerebral vascular amyloid peptide, AID(50), Alpha-CTF, Gamma-CTF(57), AICD-50
Concentration
2 mg/ml
Endotoxin level
Please contact with the lab for this information.
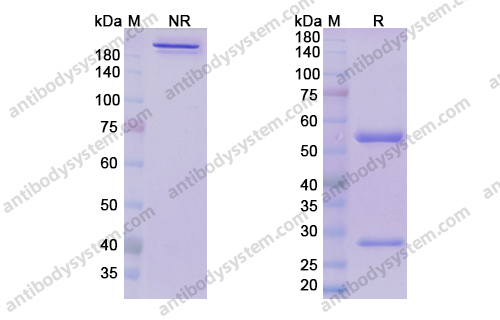
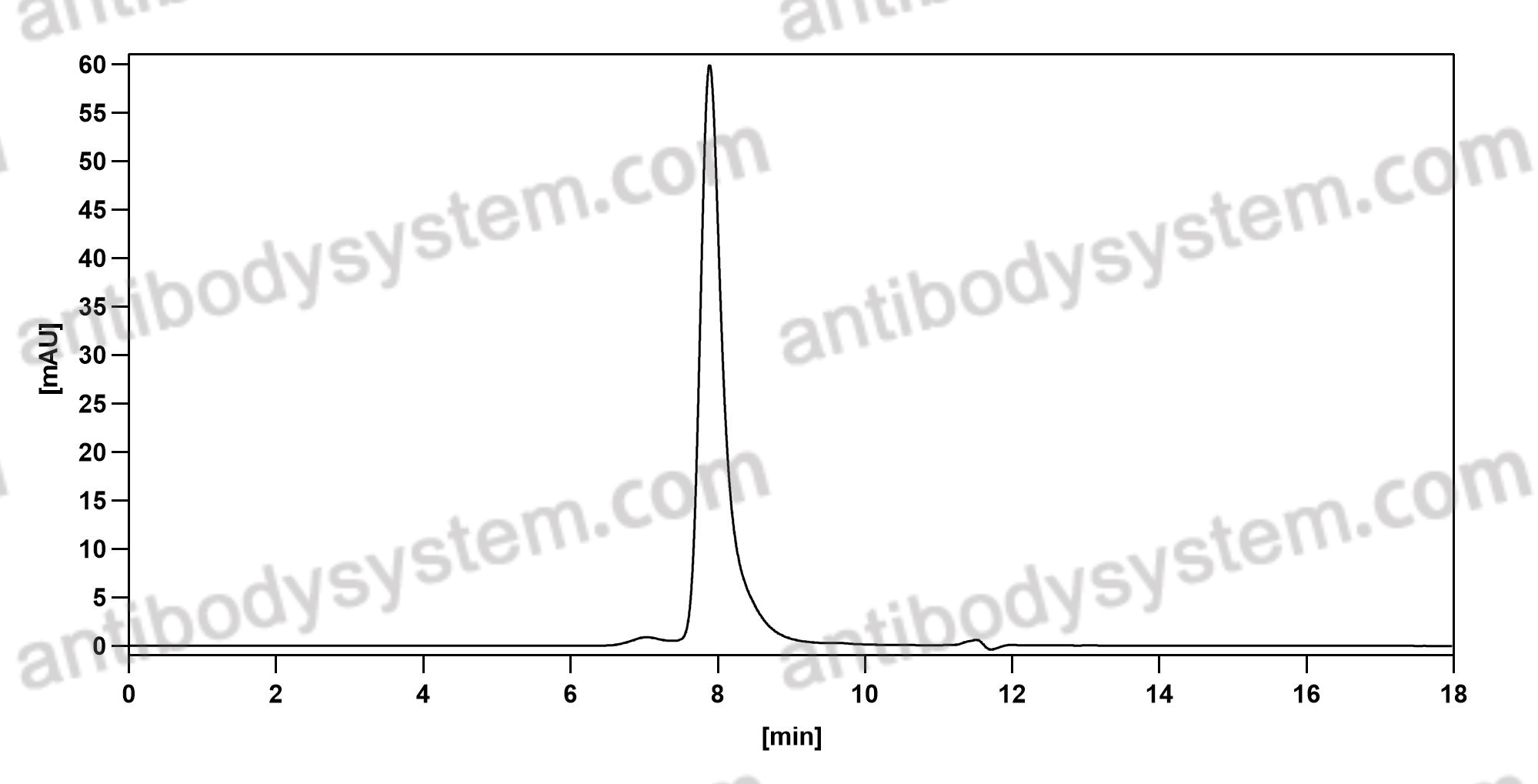
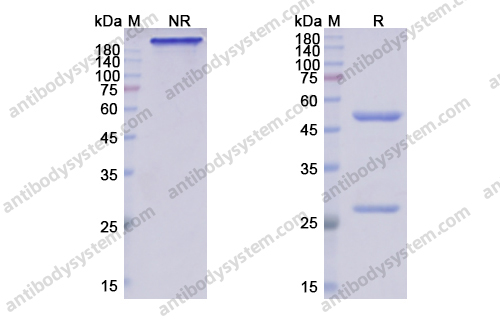
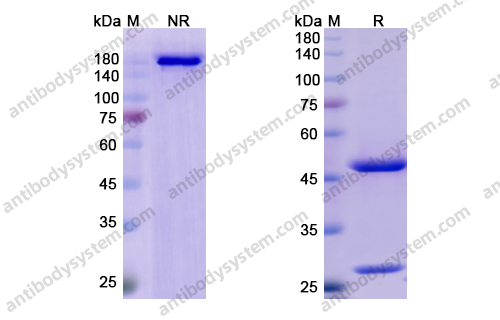
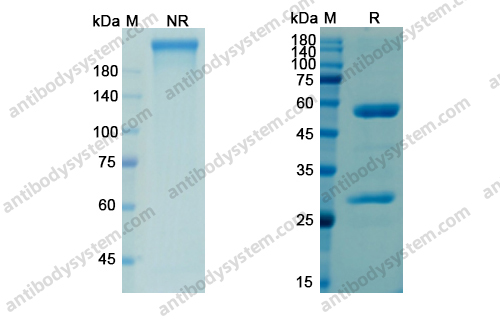
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P05067
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
LY3002813, CAS: 1931944-80-7
Clone ID
Donanemab
Donanemab in Early Alzheimer's Disease, PMID: 33720637
Donanemab in Early Alzheimer's Disease, PMID: 34379933
Donanemab (LY3002813) dose-escalation study in Alzheimer's disease, PMID: 33614890
Donanemab in Early Alzheimer's Disease. Reply, PMID: 34379934
Brain volume loss due to donanemab, PMID: 34224184
Still grasping at straws: donanemab in Alzheimer's disease, PMID: 34162295
Critical Appraisal of Amyloid Lowering Agents in AD, PMID: 34110536
Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer's Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression, PMID: 34198582
Aducanumab produced a clinically meaningful benefit in association with amyloid lowering, PMID: 33971962
Current advances and unmet needs in Alzheimer's disease trials for individuals with Down syndrome: Navigating new therapeutic frontiers., PMID:40528298
Antiamyloid treatment for dementia: concerns outweigh hopes., PMID:40511512
Comparison between treatment-placebo difference and saved time measures to assess treatment effect in Alzheimer's disease., PMID:40510745
Translational Medicine in Alzheimer's Disease: The Journey of Donanemab From Discovery to Clinical Application., PMID:40486952
Anti-amyloid antibody equilibrium binding to Aβ aggregates from human Alzheimer disease brain., PMID:40475543
Comparisons of efficacy and safety of immunotherapies for Alzheimer's disease treatment: a network meta-analysis of randomized controlled trials., PMID:40473116
MRI protocols and sequences for amyloid-related imaging abnormalities monitoring in Alzheimer's disease patients treated with monoclonal antibodies., PMID:40466018
Therapeutic time window of disease-modifying therapy for early Alzheimer's disease., PMID:40453977
Chimeric antigen receptors discriminate between tau and distinct amyloid-beta species., PMID:40448101
A 2025 update on treatment strategies for the Alzheimer's disease spectrum., PMID:40442885
Insights into pathophysiology, biomarkers, and therapeutics in tauopathies: Proceedings of the Tau2024 Global Conference., PMID:40437880
Evaluating the Cost-Effectiveness of Pharmacological Therapy in Alzheimer Disease in Brazil., PMID:40435868
[Prospects for treating Alzheimer's disease]., PMID:40420451
Anti-amyloid immunotherapies for Alzheimer's disease: Administration, side effects, and overall framework., PMID:40413817
TRAILBLAZER-ALZ 4: A phase 3 trial comparing donanemab with aducanumab on amyloid plaque clearance in early, symptomatic Alzheimer's disease., PMID:40390253
Comparative efficacy, tolerability, and acceptability of aducanumab, lecanemab, and donanemab with repetitive transcranial magnetic stimulation on cognitive function in mild cognitive impairment and Alzheimer's disease: A systematic review and network meta-analysis., PMID:40386876
Regulatory asymmetry in Alzheimer's disease care., PMID:40381642
Safety, Tolerability, and Pharmacokinetics of Donanemab in Healthy Chinese Participants: A Phase 1, Randomized, Double-Blind, Placebo-Controlled Study., PMID:40377397
Clinical evaluation of medicines for patients with mild cognitive impairment and mild dementia due to Alzheimer's disease in Japan., PMID:40370495
Alzheimer's Is a Multiform Disease of Sustained Neuronal Integrated Stress Response Driven by the C99 Fragment Generated Independently of AβPP; Proteolytic Production of Aβ Is Suppressed in AD-Affected Neurons: Evolution of a Theory., PMID:40362488
Lecanemab Treatment in a Specialty Memory Clinic., PMID:40354064
The Efficacy of Anti-amyloid Monoclonal Antibodies in Early Alzheimer's Dementia: A Systematic Review., PMID:40346804
Monoclonal antibodies against beta-amyloid protein (lecanemab and donanemab) should not be used in the treatment of Alzheimer's disease., PMID:40345230
Amyloid immunotherapy for Alzheimer's disease: the case for cautious adoption., PMID:40345229
Use of Model-Based Meta-Analysis to Inform the Design of Early Clinical Trials of Anti-Amyloid Beta Therapies in Alzheimer's Disease., PMID:40344388
FDA vs. EMA: evaluating donanemab and the global debate on accelerated approvals., PMID:40327104
The regulatory rollercoaster continues-EMA refuses donanemab., PMID:40324447
Antiamyloid Monoclonal Antibodies in Alzheimer's Disease, Part 1: Patient Selection., PMID:40320852
The efficacy and safety of anti-amyloid monoclonal antibody versus acetylcholinesterase inhibitor with an in-depth analysis across genotypes and disease stages: a systematic review and meta-analysis., PMID:40316479
Donanemab eligibility in early Alzheimer's disease: A real-world study., PMID:40313052
Re-evaluation of the efficacy and safety of anti-Aβ monoclonal antibodies (lecanemab/donanemab) in the treatment of early Alzheimer's disease., PMID:40308765
New drugs approved by the NMPA in 2024: Synthesis and clinical applications., PMID:40262297
Lecanemab preferentially binds to smaller aggregates present at early Alzheimer's disease., PMID:40237235
Data-Driven Modeling of Amyloid-beta Targeted Antibodies for Alzheimer's Disease., PMID:40235513
Clinical efficacy of anti-amyloid antibodies in apolipoprotein E ε4 homozygotes: A Bayesian reanalysis of lecanemab and donanemab phase 3 results., PMID:40225236
Clinical Benefits and Risks of Antiamyloid Antibodies in Sporadic Alzheimer Disease: Systematic Review and Network Meta-Analysis With a Web Application., PMID:40194268
Modified titration of donanemab reduces ARIA risk and maintains amyloid reduction., PMID:40172303
Donanemab: Appropriate use recommendations., PMID:40155270
Generalizability of trial criteria on amyloid-lowering therapy against Alzheimer's disease to individuals with mild cognitive impairment or early Alzheimer's disease in the general population., PMID:40122980
Maximizing the benefit and managing the risk of anti-amyloid monoclonal antibody therapy for Alzheimer's disease: Strategies and research directions., PMID:40118715
Cost-effectiveness of diagnosing and treating patients with early Alzheimer's disease with anti-amyloid treatment in a clinical setting., PMID:40111937
Lecanemab for mild Alzheimer disease - is there a way forward?, PMID:40078953
Amyloid-Related Imaging Abnormalities With Donanemab in Early Symptomatic Alzheimer Disease: Secondary Analysis of the TRAILBLAZER-ALZ and ALZ 2 Randomized Clinical Trials., PMID:40063015
Lecanemab and donanemab: NICE reconsiders controversial Alzheimer's drugs., PMID:40054859
Patient eligibility for amyloid-targeting immunotherapies in Alzheimer's disease., PMID:40011174
Targeting Amyloid Pathology in Early Alzheimer's: The Promise of Donanemab-Azbt., PMID:39998021
Amyloid-β Clearance with Monoclonal Antibodies: Transforming Alzheimer's Treatment., PMID:39980294
Chimeric Antigen Receptors Discriminate Between Tau and Distinct Amyloid-Beta Species., PMID:39974919
Eligibility for donanemab trial in a population-based study of cognitive aging., PMID:39966022
Therapeutic delivery - industry update covering October 2024., PMID:39961726