Catalog No.
DHC82803
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-lambda
Clonality
Monoclonal
Target
Extrinsic pathway inhibitor, Lipoprotein-associated coagulation inhibitor, TFPI, EPI, LACI, TFPI1, Tissue factor pathway inhibitor
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
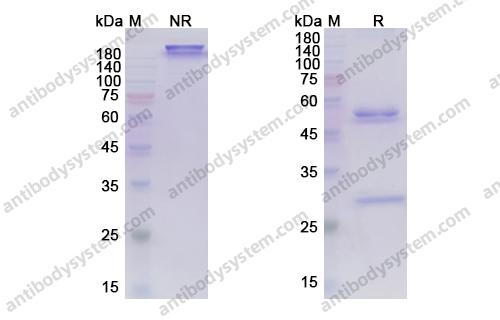
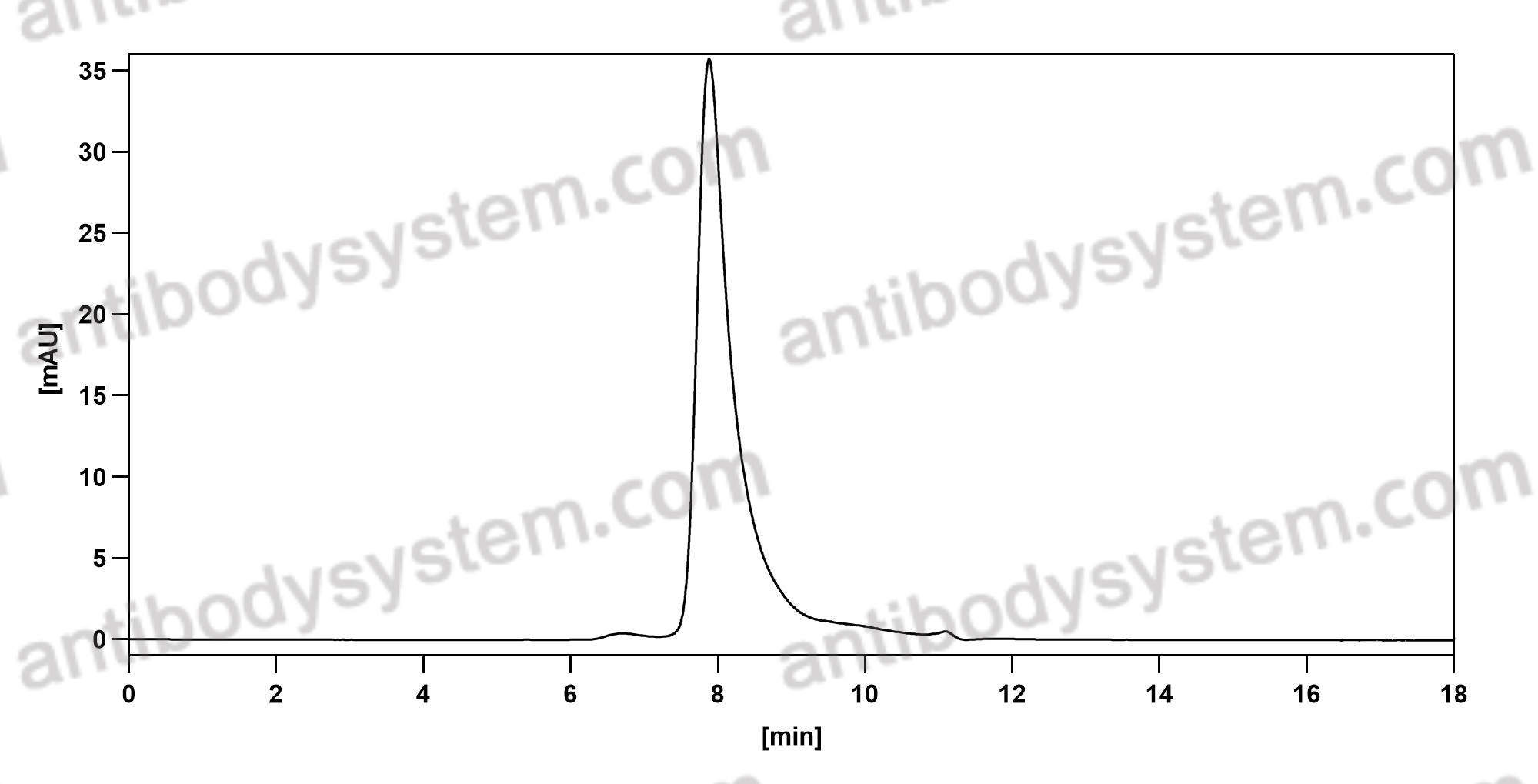
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P10646
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
PF-06741086, CAS: 1985638-39-8
Clone ID
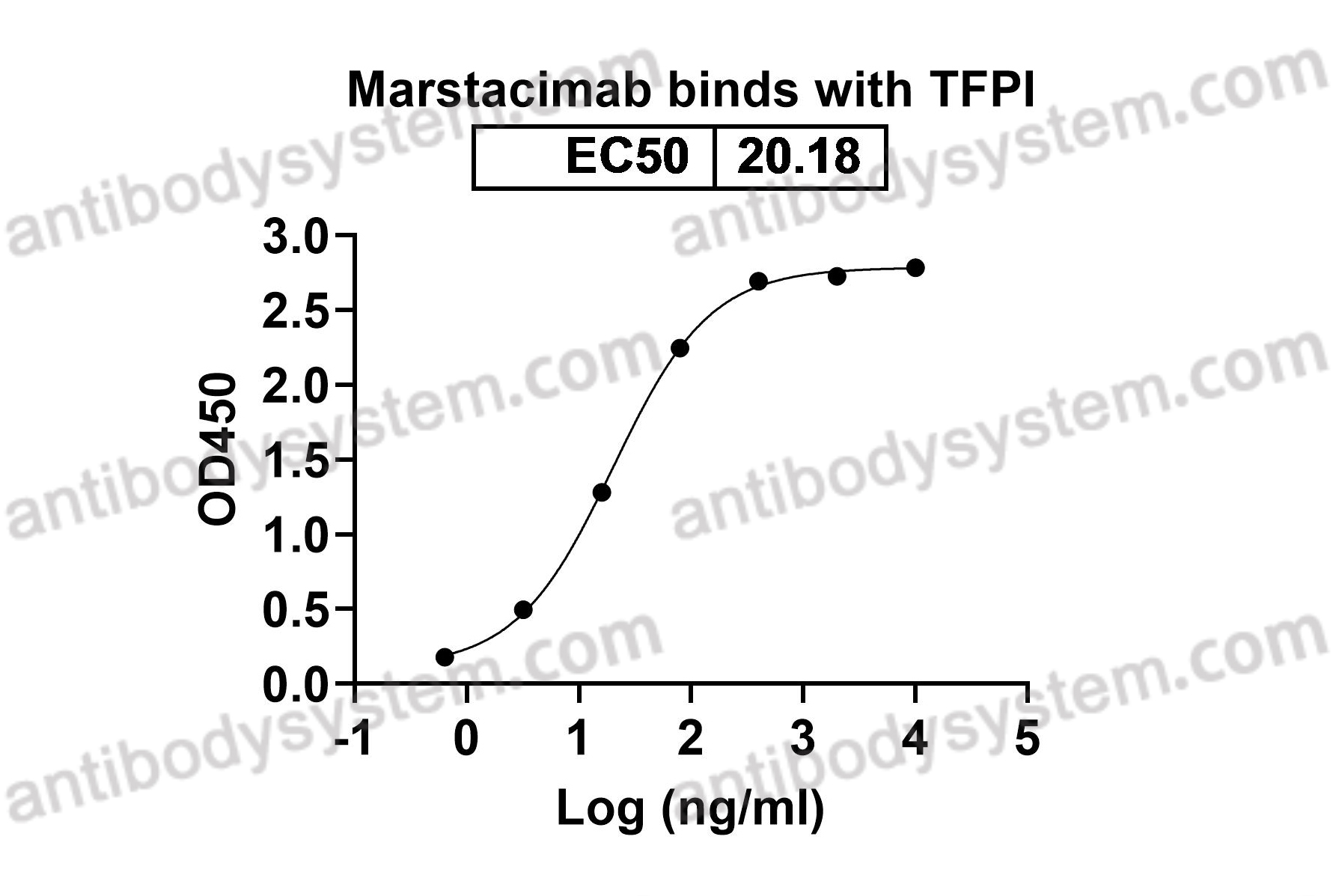
Marstacimab
Marstacimab, a tissue factor pathway inhibitor neutralizing antibody, improves coagulation parameters of ex vivo dosed haemophilic blood and plasmas, PMID: 31336410
Progress in the Development of Anti-tissue Factor Pathway Inhibitors for Haemophilia Management, PMID: 34026796
Neutralizing Antibody Assay Development with High Drug and Target Tolerance to Support Clinical Development of an Anti-TFPI Therapeutic Monoclonal Antibody, PMID: 30927117
A first-in-human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of PF-06741086, an anti-tissue factor pathway inhibitor mAb, in healthy volunteers, PMID: 29908043
Safety and efficacy of marstacimab in patients with hemophilia A and B: a systematic review and meta-analysis., PMID:40528319
Anti-tissue factor pathway inhibitors for hemophilia: are these treatments the answer to overcoming current treatment limitations?, PMID:40515600
Concizumab (Alhemo) for hemophilia A and B with inhibitors., PMID:40324964
Revolutionizing Treatment Strategies through Inhibition of Tissue Factor Pathway Inhibitor: A Promising Therapeutic Approach for Hemophilia Management., PMID:40200623
Consistent clinical factor VIII equivalency is unlikely for non-factor therapies in hemophilic mice., PMID:40176760
Novel drugs approved by the EMA, the FDA and the MHRA in 2024: A year in review., PMID:39971274
Marstacimab (Hympavzi) for hemophilia A and B., PMID:39752723
Marstacimab-hncq., PMID:39719032
Marstacimab: First Approval., PMID:39715914
Advances in Development of Drug Treatment for Hemophilia with Inhibitors., PMID:39698264
Hympavzi (Marstacimab-hncq)., PMID:39694756
Non-factor Therapies for Hemophilia: Achievements and Perspectives., PMID:39613145
Benefits and risks of non-factor therapies: Redefining haemophilia treatment goals in the era of new technologies., PMID:38481077
Non-clotting factor therapies for preventing bleeds in people with congenital hemophilia A or B., PMID:38411279
Evolution of Antidrug Antibody Assays During the Development of Anti-Tissue Factor Pathway Inhibitor Monoclonal Antibody Marstacimab., PMID:37610502
Safety, tolerability, pharmacokinetics and pharmacodynamics of a single dose of marstacimab in Chinese participants with severe haemophilia., PMID:37339017
The Use of Bypassing Treatment Strategies in Hemophilia and Their Effect on Laboratory Testing., PMID:37146647
Long-term safety and efficacy of the anti-tissue factor pathway inhibitor marstacimab in participants with severe haemophilia: Phase II study results., PMID:36220152
A phase 1b/2 clinical study of marstacimab, targeting human tissue factor pathway inhibitor, in haemophilia., PMID:35999026
The Vascular Endothelium and Coagulation: Homeostasis, Disease, and Treatment, with a Focus on the Von Willebrand Factor and Factors VIII and V., PMID:35955419
Current and future therapies for haemophilia-Beyond factor replacement therapies., PMID:35869698
Anti-TFPI for hemostasis induction in patients with rare bleeding disorders, an ex vivo thrombin generation (TG) guided pilot study., PMID:35525014
Hemostatic efficacy of marstacimab alone or in combination with bypassing agents in hemophilia plasmas and a mouse bleeding model., PMID:35316941
Progress in the Development of Anti-tissue Factor Pathway Inhibitors for Haemophilia Management., PMID:34026796
Practical considerations for nonfactor-replacement therapies in the treatment of haemophilia with inhibitors., PMID:33742707
Marstacimab, a tissue factor pathway inhibitor neutralizing antibody, improves coagulation parameters of ex vivo dosed haemophilic blood and plasmas., PMID:31336410
Neutralizing Antibody Assay Development with High Drug and Target Tolerance to Support Clinical Development of an Anti-TFPI Therapeutic Monoclonal Antibody., PMID:30927117
A first-in-human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of PF-06741086, an anti-tissue factor pathway inhibitor mAb, in healthy volunteers., PMID:29908043