Catalog No.
DHB88201
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-lambda
Clonality
Monoclonal
Target
F12, HAF, Hageman factor, Beta-factor XIIa part 2, Coagulation factor XII
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
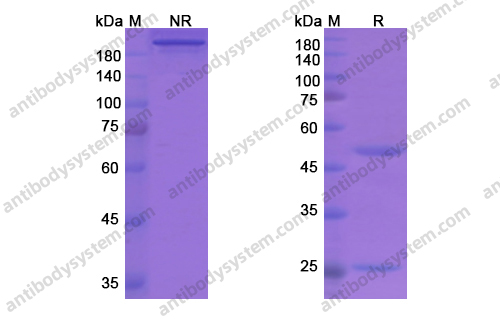
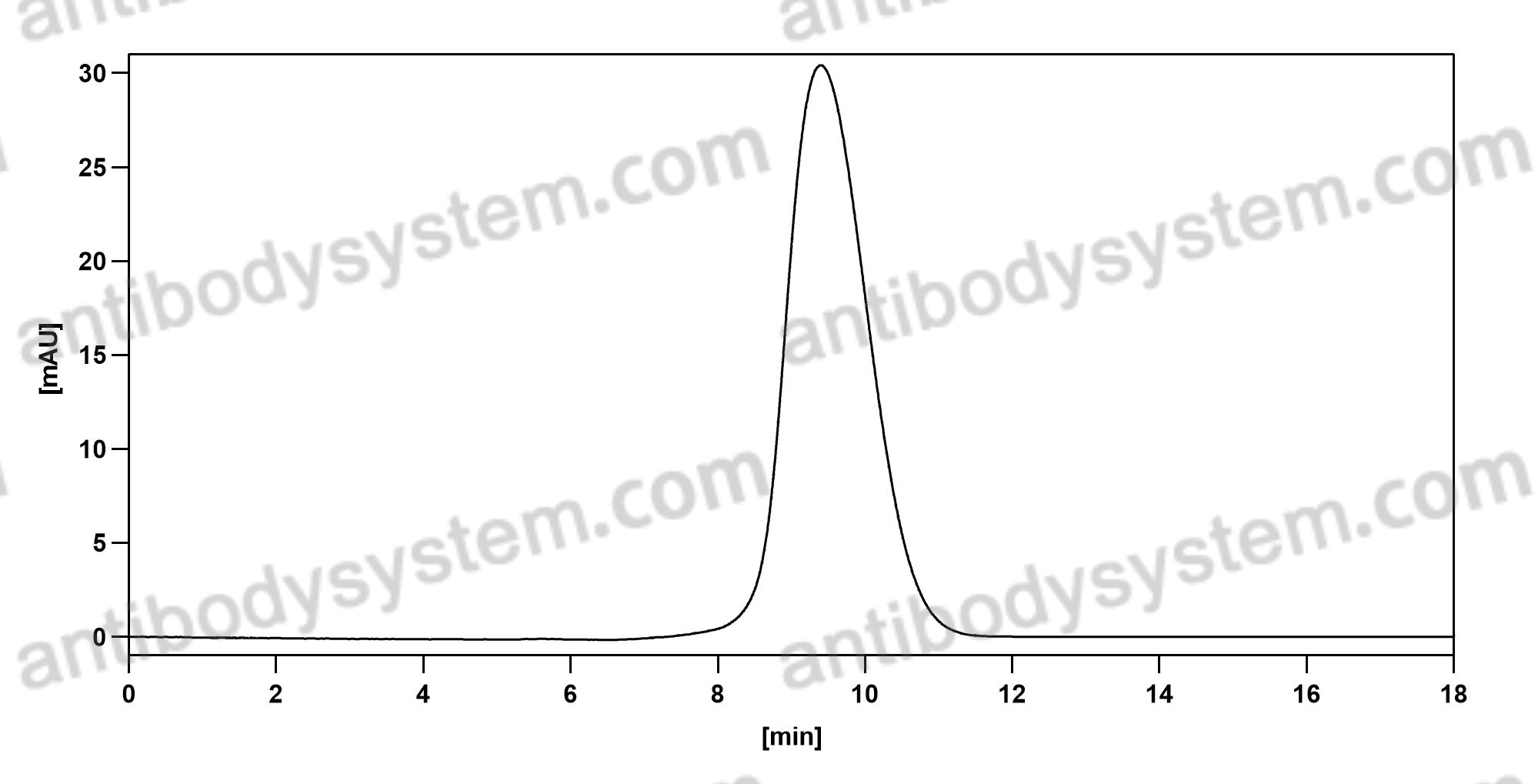
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P00748
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
CSL312, CAS: 2162134-62-3
Clone ID
Garadacimab
Network Meta-Analysis of Pharmacological Therapies for Long-Term Prophylactic Treatment of Patients with Hereditary Angioedema., PMID:40434599
The potential of factor XII inhibitors in preventing hereditary angioedema attacks., PMID:40411772
Garadacimab improves long-term health-related quality of life in patients with hereditary angioedema., PMID:40380363
Becoming attack-free further improves health-related quality of life in patients with hereditary angioedema receiving garadacimab., PMID:40380356
Garadacimab: First Approval., PMID:40261472
Population Pharmacokinetic/Pharmacodynamic and Exposure-Response Modeling of Garadacimab in Healthy Volunteers and Patients With Hereditary Angioedema., PMID:40042097
Unravelling the impact of SARS-CoV-2 on hemostatic and complement systems: a systems immunology perspective., PMID:39885991
Pharmacokinetics, Pharmacodynamics, and Safety of Subcutaneous and Intravenous Garadacimab Following Single-Dose Administration in Healthy Japanese and White Adults., PMID:39582204
The international HAE guideline under real-life conditions: From possibilities to limits in daily life - current real-world data of 8 German angioedema centers., PMID:39564138
Long-term safety and efficacy of garadacimab for preventing hereditary angioedema attacks: Phase 3 open-label extension study., PMID:39370961
Timing of Onset of Garadacimab for Preventing Hereditary Angioedema Attacks., PMID:39353415
Targeting factor XIIa for therapeutic interference with hereditary angioedema., PMID:39331688
Structural basis for the inhibition of βFXIIa by garadacimab., PMID:39059382
[Clinical development of anti-FXII therapeutics as a target in thrombo-inflammation]., PMID:38845252
Garadacimab for hereditary angioedema attack prevention: long-term efficacy, quality of life, and safety data from a phase 2, randomised, open-label extension study., PMID:38710185
Long-term prophylaxis for hereditary angioedema: Initial experiences with garadacimab and lanadelumab., PMID:38024849
Efficacy and Safety of Garadacimab in Combination with Standard of Care Treatment in Patients with Severe COVID-19., PMID:37000214
Efficacy and safety of garadacimab, a factor XIIa inhibitor for hereditary angioedema prevention (VANGUARD): a global, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial., PMID:36868261
HDX-MS study on garadacimab binding to activated FXII reveals potential binding interfaces through differential solvent exposure., PMID:36628468
Fit-for-purpose validation of a drug-tolerant immunogenicity assay for a human mAb drug in animal safety studies., PMID:36526009
Treatment of hereditary angioedema-single or multiple pathways to the rescue., PMID:36172291
Prophylactic use of an anti-activated factor XII monoclonal antibody, garadacimab, for patients with C1-esterase inhibitor-deficient hereditary angioedema: a randomised, double-blind, placebo-controlled, phase 2 trial., PMID:35219377
A phase I, first-in-human, randomized dose-escalation study of anti-activated factor XII monoclonal antibody garadacimab., PMID:34859955
Pharmacokinetic/pharmacodynamic modeling for dose selection for the first-in-human trial of the activated Factor XII inhibitor garadacimab (CSL312)., PMID:34811931
Factor XII/XIIa inhibitors: Their discovery, development, and potential indications., PMID:32883641