Catalog No.
DHB90006
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
CPAMD4, C5, C3 and PZP-like alpha-2-macroglobulin domain-containing protein 4, Complement C5
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
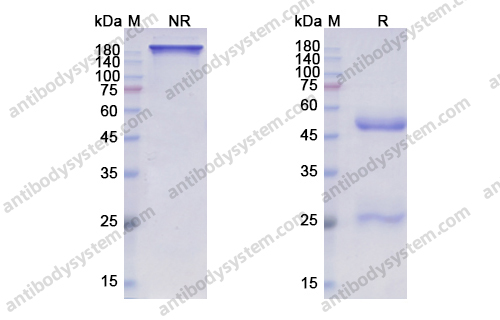
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P01031
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
REGN-3918, CAS: 2096328-94-6
Clone ID
Pozelimab
Pharmacological Insights on USFDA-Approved Novel Drug Therapies in the Year 2023., PMID:40464188
The Role of Complement in the Pathogenesis and Treatment of Myasthenia Gravis., PMID:40422242
Complement dysregulation at lymphatics., PMID:40300720
The patient experience of CHAPLE disease: results from interviews conducted as part of a clinical trial for an ultra-rare condition., PMID:39934837
A Pedigree With Complement Hyperactivation, Angiopathic Thrombosis, and Severe Protein-losing Enteropathy (CHAPLE) Disease: Variable Penetrance and Treatment With Pozelimab., PMID:39689772
An evaluation of pozelimab for the treatment of CHAPLE disease., PMID:39657032
Population pharmacokinetic analyses of pozelimab in patients with CD55-deficient protein-losing enteropathy (CHAPLE disease)., PMID:39349796
Pozelimab for CHAPLE disease: results from in-trial interviews and clinical outcome assessments., PMID:39118150
Pharmacotherapy for CD55 deficiency with CHAPLE disease: how close are we to a cure?, PMID:39092479
Complement inhibition in paroxysmal nocturnal hemoglobinuria: From biology to therapy., PMID:38622956
Pozelimab, a human monoclonal immunoglobulin for the treatment of CHAPLE disease., PMID:38598858
Evaluating the efficacy and safety of pozelimab in patients with CD55 deficiency with hyperactivation of complement, angiopathic thrombosis, and protein-losing enteropathy disease: an open-label phase 2 and 3 study., PMID:38278170
A new drug for rare diseases: pozelimab for CHAPLE disease., PMID:38278169
Antibodies to watch in 2024., PMID:38178784
Pozelimab: First Approval., PMID:37856038
Pozelimab-bbfg., PMID:37769306
Characterization of multivalent complexes formed in the presence of more than one conventional antibody to terminal complement component C5., PMID:37079521
Pharmacokinetics and pharmacodynamics of pozelimab alone or in combination with cemdisiran in non-human primates., PMID:35709087
Paroxysmal nocturnal hemoglobinuria: advances in the understanding of pathophysiology, diagnosis, and treatment., PMID:35699625
CD55-deficiency in Jews of Bukharan descent is caused by the Cromer blood type Dr(a-) variant., PMID:35314883
Inhibition of complement pathway activation with Pozelimab, a fully human antibody to complement component C5., PMID:32384086