Catalog No.
DHB90005
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
CPAMD4, C5, C3 and PZP-like alpha-2-macroglobulin domain-containing protein 4, Complement C5
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
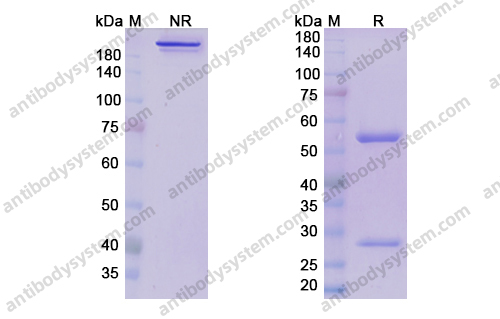
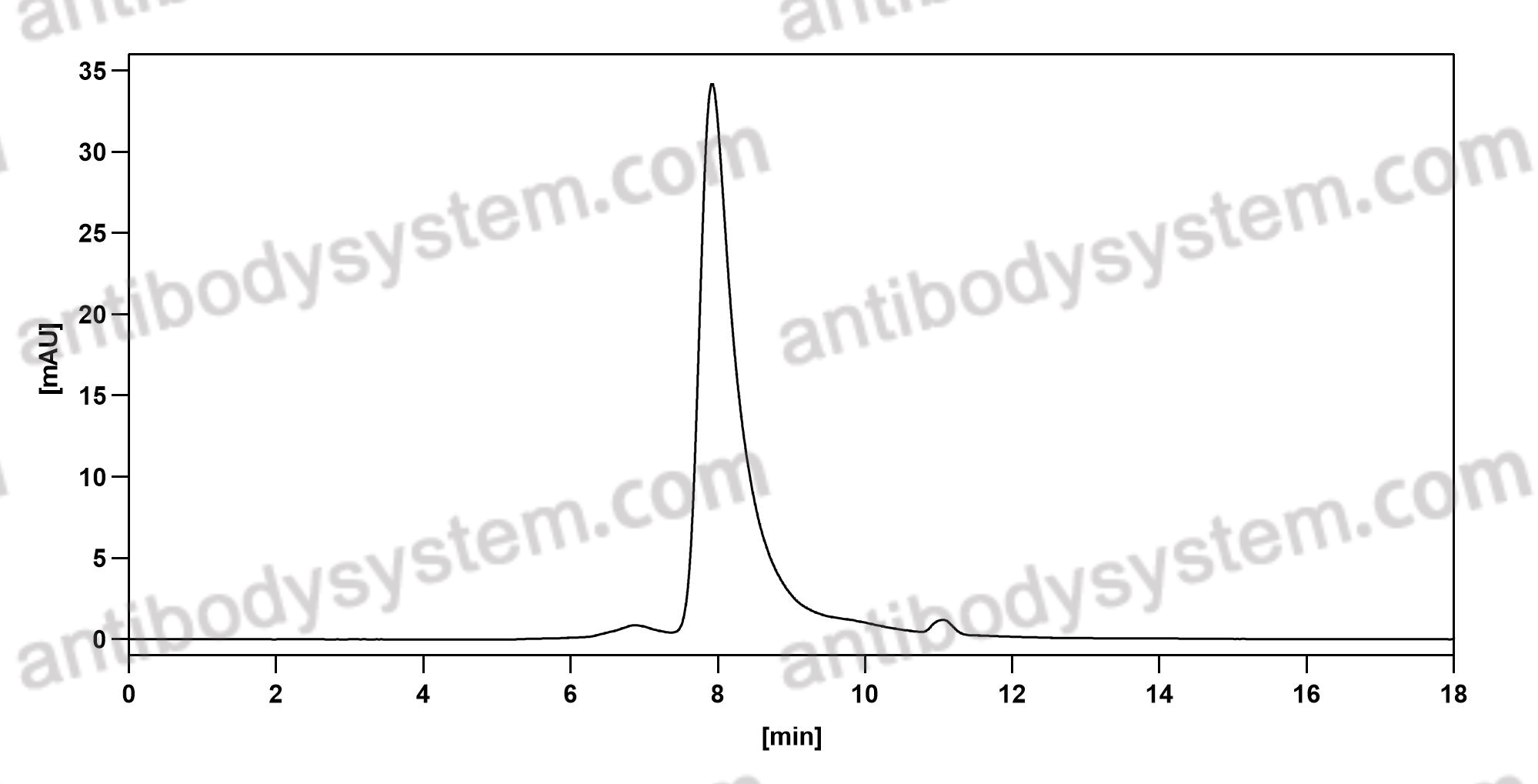
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P01031
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
RG-6107, RO7112689, SKY-59, CAS: 1917321-26-6
Clone ID
Crovalimab
The complement C5 inhibitor crovalimab in paroxysmal nocturnal hemoglobinuria, PMID: 31978221
[Antibody therapy for paroxysmal nocturnal hemoglobinuria], PMID: 32908057
[Novel therapeutics for paroxysmal nocturnal hemoglobinuria], PMID: 34108330
An interview with Nancy Valente, the Global Head of Hematology Development at Roche, PMID: 32684001
[Current treatment of paroxysmal nocturnal hemoglobinuria and prospects for new therapeutic agents in the future], PMID: 34108318
Patient-reported outcomes in patients with paroxysmal nocturnal hemoglobinuria treated with crovalimab and approved C5 inhibitors in the phase III COMMODORE 2 and 1 studies., PMID:40515823
Advancements in Complement Inhibition for PNH and Primary Complement Mediated Thrombotic Microangiopathy., PMID:40354320
The Advancing Landscape of Paroxysmal Nocturnal Hemoglobinuria Treatment., PMID:40257298
Breakthrough hemolysis in paroxysmal nocturnal hemoglobinuria throughout clinical trials: from definition to clinical practice., PMID:40233322
Crovalimab: a new era in paroxysmal nocturnal hemoglobinuria management., PMID:40110253
FDA approval of crovalimab: a milestone in paroxysmal nocturnal hemoglobinuria treatment., PMID:40109627
Current status and perspectives of hematopoietic cell transplantation in patients with paroxysmal nocturnal hemoglobinuria., PMID:39840046
Pharmacokinetic characterization and exposure-response relationship of crovalimab in the COMMODORE 1, 2 and 3 and COMPOSER trials of patients with paroxysmal nocturnal haemoglobinuria., PMID:39835421
Advancements in PNH treatment: crovalimab's clinical efficacy., PMID:39649925
Crovalimab in the paroxysmal nocturnal hemoglobinuria treatment landscape., PMID:39620653
Safety of Crovalimab Versus Eculizumab in Patients With Paroxysmal Nocturnal Haemoglobinuria (PNH): Pooled Results From the Phase 3 COMMODORE Studies., PMID:39535306
Beyond Recycling Antibodies: Crovalimab's Molecular Design Enables Four-Weekly Subcutaneous Injections for PNH Treatment., PMID:39519232
[New treatment strategies for paroxysmal nocturnal hemoglobinuria: the guideline update and novel complement-targeting drugs]., PMID:39505557
Navigating the Complement Pathway to Optimize PNH Treatment with Pegcetacoplan and Other Currently Approved Complement Inhibitors., PMID:39273426
Crovalimab-akkz., PMID:39271132
A Cell-Based Assay to Measure the Activity of the Complement Convertases., PMID:39081762
Phase 3 randomized COMMODORE 1 trial: Crovalimab versus eculizumab in complement inhibitor-experienced patients with paroxysmal nocturnal hemoglobinuria., PMID:38924124
Phase 3 randomized COMMODORE 2 trial: Crovalimab versus eculizumab in patients with paroxysmal nocturnal hemoglobinuria naive to complement inhibition., PMID:38884175
Crovalimab: First Approval., PMID:38740735
Complement inhibition in paroxysmal nocturnal hemoglobinuria: From biology to therapy., PMID:38622956
Complement inhibitors in pediatric kidney diseases: new therapeutic opportunities., PMID:37733095
Efficacy and safety of the C5 inhibitor crovalimab in complement inhibitor-naive patients with PNH (COMMODORE 3): A multicenter, Phase 3, single-arm study., PMID:37421604
Crovalimab treatment in patients with paroxysmal nocturnal haemoglobinuria: Long-term results from the phase I/II COMPOSER trial., PMID:37321625
Complement Inhibitors in the Management of Complement-Mediated Hemolytic Uremic Syndrome and Paroxysmal Nocturnal Hemoglobinuria., PMID:37104648
Characterization of multivalent complexes formed in the presence of more than one conventional antibody to terminal complement component C5., PMID:37079521
[COVID-19 development during the treatment of paroxysmal nocturnal hemoglobinuria]., PMID:37019678
Mitigating Drug-Target-Drug Complexes in Patients With Paroxysmal Nocturnal Hemoglobinuria Who Switch C5 Inhibitors., PMID:36660902
Validation of a method to analyze size distribution of crovalimab-complement C5-eculizumab complexes in human serum., PMID:35904159
Complement System as a New Target for Hematopoietic Stem Cell Transplantation-Related Thrombotic Microangiopathy., PMID:35890144
Paroxysmal nocturnal hemoglobinuria: advances in the understanding of pathophysiology, diagnosis, and treatment., PMID:35699625
Crovalimab for treatment of patients with paroxysmal nocturnal haemoglobinuria and complement C5 polymorphism: Subanalysis of the phase 1/2 COMPOSER study., PMID:35608260
[Novel therapeutics for paroxysmal nocturnal hemoglobinuria]., PMID:34108330
[Current treatment of paroxysmal nocturnal hemoglobinuria and prospects for new therapeutic agents in the future]., PMID:34108318
[Antibody therapy for paroxysmal nocturnal hemoglobinuria]., PMID:32908057
An interview with Nancy Valente, the Global Head of Hematology Development at Roche., PMID:32684001
The complement C5 inhibitor crovalimab in paroxysmal nocturnal hemoglobinuria., PMID:31978221