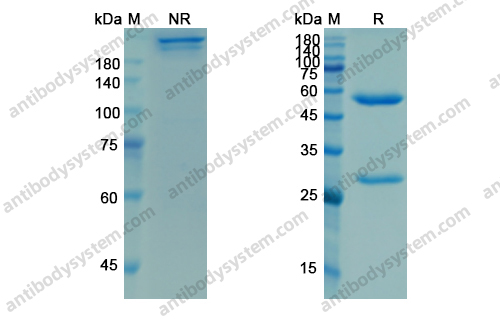
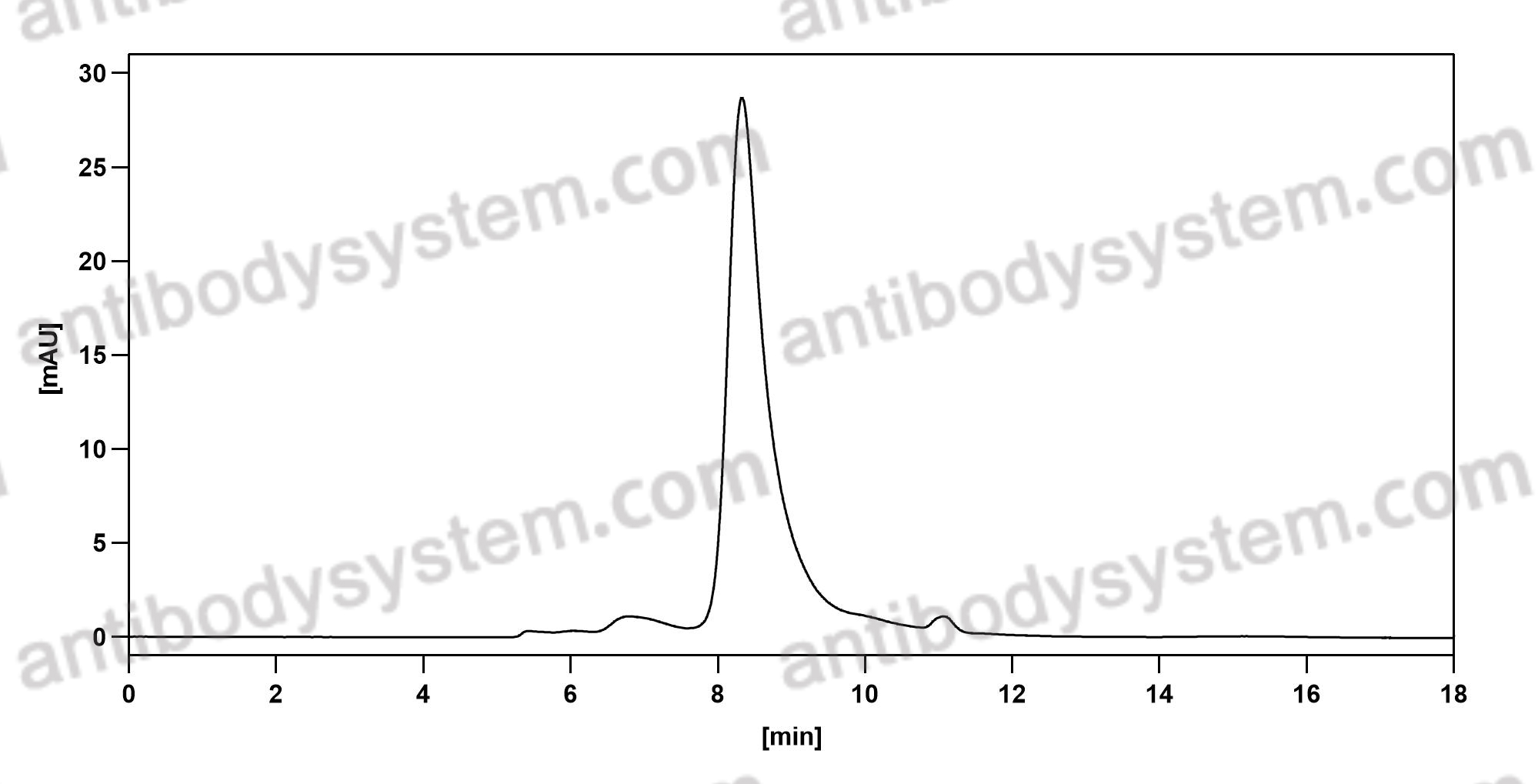
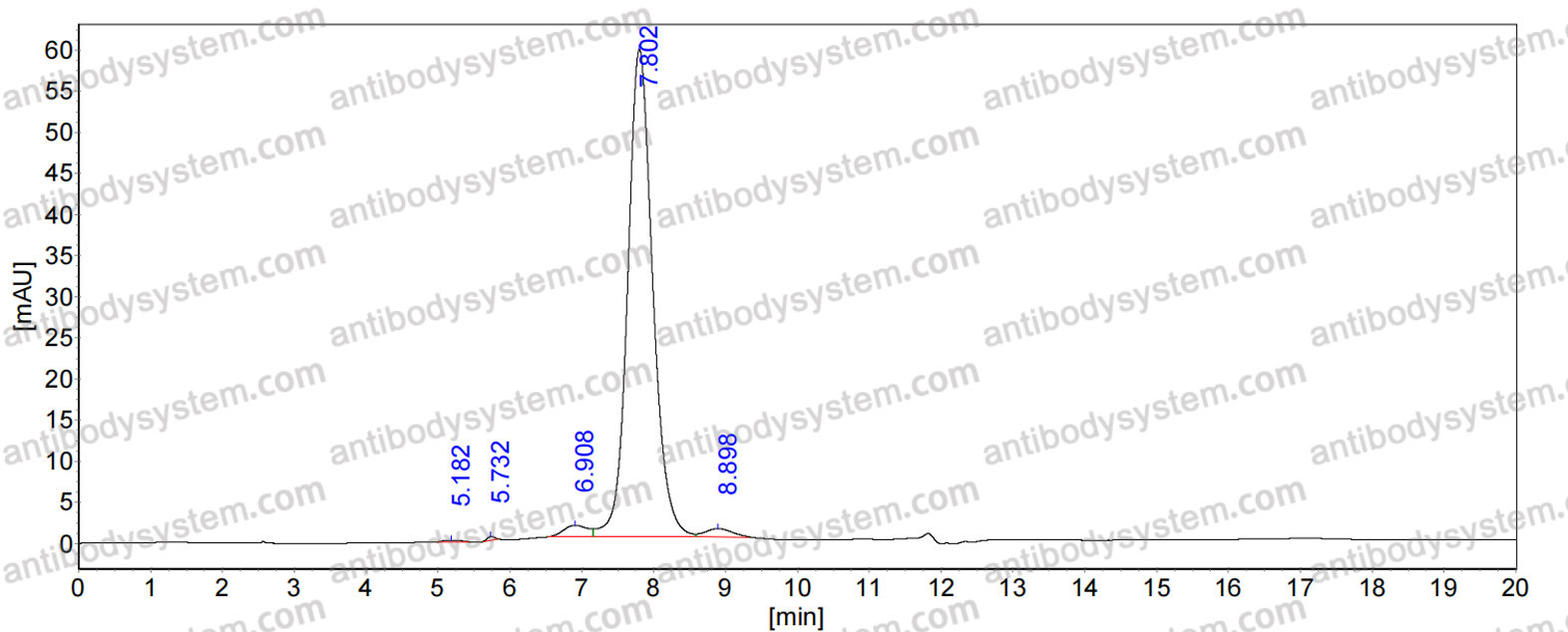
Ligelizumab for Chronic Spontaneous Urticaria, PMID: 31577874
The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab, PMID: 31913280
Ligelizumab for the treatment of chronic spontaneous urticaria, PMID: 32380864
Ligelizumab for Chronic Spontaneous Urticaria, PMID: 32023385
Ligelizumab for chronic spontaneous urticaria, PMID: 32002982
Ligelizumab for Chronic Spontaneous Urticaria, PMID: 32023384
Ligelizumab for Chronic Spontaneous Urticaria. Reply, PMID: 32023387
Ligelizumab for Chronic Spontaneous Urticaria. Reply, PMID: 32023386
Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects, PMID: 25200415
Ligelizumab Is Superior to Omalizumab for Chronic Spontaneous Urticaria, PMID: 32208012
New treatments for chronic urticaria, PMID: 31446134
Antibodies to watch in 2021, PMID: 33459118
Current and emerging treatments for chronic spontaneous urticaria, PMID: 31494233
Structure of intact IgE and the mechanism of ligelizumab revealed by electron microscopy, PMID: 32037590
High-Dose Omalizumab versus Ligelizumab for the Treatment of Chronic Spontaneous Urticaria: Do Not We Need a Head-To-Head Comparison?, PMID: 33321510
Ligelizumab treatment for severe asthma: learnings from the clinical development programme, PMID: 33747510
Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses, PMID: 27185571
Novel Therapeutic Approaches and Targets for Treatment of Chronic Urticaria: New Insights and Promising Targets for a Challenging Disease, PMID: 32603278
Tailored therapy for severe asthma, PMID: 25671117
New biologics in the treatment of urticaria, PMID: 30015639
A Better IgE Trap to Control Urticaria, PMID: 31577881
Looking forward to new targeted treatments for chronic spontaneous urticaria, PMID: 28078079
An Update on Anti-IgE Therapy in Pediatric Respiratory Diseases, PMID: 29290750
Rapid desensitization of humanized mice with anti-human FcεRIα monoclonal antibodies, PMID: 31836406
Emerging Biomarkers and Therapeutic Pipelines for Chronic Spontaneous Urticaria, PMID: 30033912
Anti-IgE for the Treatment of Chronic Urticaria, PMID: 33628747
Evaluation of Pharmacologic Treatments for H1 Antihistamine-Refractory Chronic Spontaneous Urticaria: A Systematic Review and Network Meta-analysis, PMID: 34431983
Advances in asthma in 2016: Designing individualized approaches to management, PMID: 28709967
The Intersection of IgE Autoantibodies and Eosinophilia in the Pathogenesis of Bullous Pemphigoid, PMID: 31636640
The use of biologics in food allergy, PMID: 33966304
Biologics for the Use in Chronic Spontaneous Urticaria: When and Which, PMID: 33685605
A Comprehensive Approach to Urticaria: From Clinical Presentation to Modern Biological Treatments Through Pathogenesis, PMID: 33385176
Quantitative in vitro and in vivo models to assess human IgE B cell receptor crosslinking by IgE and EMPD IgE targeting antibodies, PMID: 28647457
Management of Pediatric Chronic Spontaneous Urticaria: A Review of Current Evidence and Guidelines, PMID: 33727832
Structural and Functional Insights Into IgE Receptor Interactions and Disruptive Inhibition., PMID:40305523
Efficacy and Safety of Ligelizumab in Chronic Spontaneous Urticaria: A Systematic Review and Meta-analysis of Randomized Controlled Trials., PMID:40186041
Emerging treatments for dermatologic diseases in infants, children, and adolescents: a systematic review of clinical trials on biologics and small molecule inhibitors., PMID:40042725
From wheal to wellness: efficacy and safety of ligelizumab in chronic spontaneous urticaria: a systematic review and meta-analysis., PMID:40014098
Long term safety and efficacy of ligelizumab in the treatment of Japanese patients with chronic spontaneous urticaria., PMID:39327219
Time-course and dose-effect of omalizumab in treating chronic idiopathic urticaria/chronic spontaneous urticaria., PMID:38967658
Biologic therapy for chronic spontaneous urticaria in pediatrics and adolescents: current landscape, challenges, and future perspectives., PMID:38733124
New biologics for food allergy., PMID:38547423
Role of biologics in severe food allergy., PMID:38538153
Efficacy and safety of ligelizumab in adults and adolescents with chronic spontaneous urticaria: results of two phase 3 randomised controlled trials., PMID:38008109
Comparative efficacy of ligelizumab versus omalizumab in chronic spontaneous urticaria., PMID:38008108
Comprehensive Assessment of Pharmacokinetics, Pharmacodynamics, and Tolerability of Ligelizumab in Healthy Volunteers and Patients with Chronic Spontaneous Urticaria to Optimize Its Subcutaneous Delivery System., PMID:37765235
Autoreactive IgE: Pathogenic role and therapeutic target in autoimmune diseases., PMID:37555488
Biologics to treat anaphylaxis., PMID:37527059
Ligelizumab in adolescents with chronic spontaneous urticaria: Results of a dedicated phase 2b randomized clinical trial supported with pharmacometric analysis., PMID:37492920
Why a Complete Response Is the Treatment Aim in Chronic Spontaneous Urticaria., PMID:37240667
Anti-immunoglobulin E for food allergy., PMID:37031775
IgE Depletion with Ligelizumab Does Not Significantly Improve Clinical Symptoms in Patients with Moderate-to-Severe Atopic Dermatitis., PMID:37004878
Impact of Penicillin Allergy Label on Clinical Outcomes of Pneumonia in Children., PMID:36948494
Mapping knowledge structure and research of the biologic treatment of asthma: A bibliometric study., PMID:36845128
Successful ligelizumab treatment of severe refractory solar urticaria., PMID:36796509
Current and future management of chronic spontaneous urticaria and chronic inducible urticaria., PMID:36719690
Ligelizumab improves angioedema, disease severity and quality-of-life in patients with chronic spontaneous urticaria., PMID:36440464
Ligelizumab impairs IgE-binding to plasmacytoid dendritic cells more potently than omalizumab and restores IFN-α production and FOXP3+ Treg generation., PMID:36315052
The rationale for development of ligelizumab in food allergy., PMID:36185545
Penicillin Allergy Label Is Associated With Worse Clinical Outcomes in Bacterial Pneumonia., PMID:36182647
A case of chronic spontaneous urticaria unresponsive to ligelizumab successfully treated with omalizumab., PMID:36164841
Pathophysiology, Diagnosis, and Management of Chronic Spontaneous Urticaria: A Literature Review., PMID:36048326
Identification of mast cell progenitor cells in the airways of individuals with allergic asthma., PMID:36038252
Racial and ethnic disparities in biologic prescriptions for asthma in the United States., PMID:35995400
Hesitancy and reactogenicity to mRNA-based COVID-19 vaccines-Early experience with vaccine rollout in a multi-site healthcare system., PMID:35930586
IgE-neutralizing UB-221 mAb, distinct from omalizumab and ligelizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms., PMID:35912861
Nodular Skin Lesions in a Patient With X-Linked Agammaglobulinemia., PMID:35790498
Current and Future Approaches in Management of Chronic Spontaneous Urticaria Using Anti-IgE Antibodies., PMID:35744079
Autoimmune chronic spontaneous urticaria., PMID:35667749
Ligelizumab improves sleep interference and disease burden in patients with chronic spontaneous urticaria., PMID:35218324
Emerging treatments for chronic urticaria., PMID:35166638
[Dermatology - Latest developments in targeted therapies for atopic dermatitis, alopecia areata and chronic spontaneous urticaria]., PMID:35107888
Bispecific T-Cell Engagers Targeting Membrane-Bound IgE., PMID:34829798
Sustained safety and efficacy of ligelizumab in patients with chronic spontaneous urticaria: A one-year extension study., PMID:34773261
Impact of Pharmacological Treatments for Chronic Spontaneous Urticaria with an Inadequate Response to H1-Antihistamines on Health-Related Quality of Life: A Systematic Review and Network Meta-Analysis., PMID:34695599
Evaluation of Pharmacologic Treatments for H1 Antihistamine-Refractory Chronic Spontaneous Urticaria: A Systematic Review and Network Meta-analysis., PMID:34431983
The use of biologics in food allergy., PMID:33966304
Ligelizumab treatment for severe asthma: learnings from the clinical development programme., PMID:33747510
Management of Pediatric Chronic Spontaneous Urticaria: A Review of Current Evidence and Guidelines., PMID:33727832
Using National Registries to Identify Targeted Therapies for Refractory Urticaria., PMID:33690233
Biologics for the Use in Chronic Spontaneous Urticaria: When and Which., PMID:33685605
Anti-IgE for the Treatment of Chronic Urticaria., PMID:33628747
Antibodies to watch in 2021., PMID:33459118
A Comprehensive Approach to Urticaria: From Clinical Presentation to Modern Biological Treatments Through Pathogenesis., PMID:33385176