Catalog No.
DHJ28402
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
T-cell immunoglobulin mucin receptor 3, HAVcr-2, TIMD-3, T-cell membrane protein 3, HAVCR2, Hepatitis A virus cellular receptor 2, CD366, TIMD3, TIM-3, TIM3, T-cell immunoglobulin and mucin domain-containing protein 3
Concentration
1.56 mg/ml
Endotoxin level
Please contact with the lab for this information.
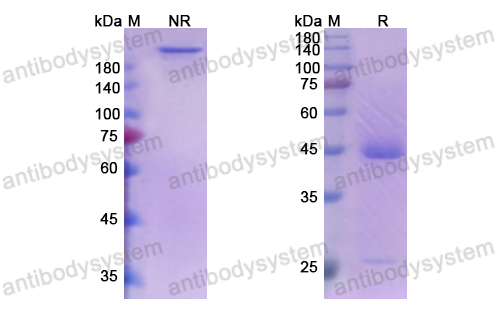
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q8TDQ0
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MBG-453, CAS: 2252262-24-9
Clone ID
Sabatolimab
Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors, PMID: 33883177
Management of patients with higher-risk myelodysplastic syndromes after failure of hypomethylating agents: What is on the horizon?, PMID: 33762100
ADORE: an open platform study of ruxolitinib in combination with other novel therapies in patients with myelofibrosis., PMID:40334082
Managing the unmanageable: evidence-driven approaches to real-world patient prototypes of TP53-mutant myelodysplastic neoplasms and acute myeloid leukemia., PMID:39349613
Correction: Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors., PMID:39224022
Sabatolimab in combination with spartalizumab in patients with non-small cell lung cancer or melanoma who received prior treatment with anti-PD-1/PD-L1 therapy: a phase 2 multicentre study., PMID:39209782
Charting the cellular biogeography in colitis reveals fibroblast trajectories and coordinated spatial remodeling., PMID:38569542
Frontline treatment options for higher-risk MDS: can we move past azacitidine?, PMID:38066872
Sabatolimab plus hypomethylating agents in previously untreated patients with higher-risk myelodysplastic syndromes (STIMULUS-MDS1): a randomised, double-blind, placebo-controlled, phase 2 trial., PMID:38065203
Phase Ib study of sabatolimab (MBG453), a novel immunotherapy targeting TIM-3 antibody, in combination with decitabine or azacitidine in high- or very high-risk myelodysplastic syndromes., PMID:37994196
Sabatolimab (MBG453) model-informed drug development for dose selection in patients with myelodysplastic syndrome/acute myeloid leukemia and solid tumors., PMID:37186155
STIMULUS-MDS2 design and rationale: a phase III trial with the anti-TIM-3 sabatolimab (MBG453) + azacitidine in higher risk MDS and CMML-2., PMID:37083373
[Management of AML in the elderly]., PMID:36870810
Correction to: Characterization of sabatolimab, a novel immunotherapy with immuno-myeloid activity directed against TIM-3 receptor., PMID:36819977
Immune Checkpoint Molecule TIGIT Regulates Kidney T Cell Functions and Contributes to AKI., PMID:36747315
Biological therapy in elderly patients with acute myeloid leukemia., PMID:36715330
Advances in myelodysplastic syndromes: promising novel agents and combination strategies., PMID:36620919
TIM-3: a tumor-associated antigen beyond checkpoint inhibition?, PMID:36406467
Updates on the Management of Acute Myeloid Leukemia., PMID:36230677
Current status of phase 3 clinical trials in high-risk myelodysplastic syndromes: pitfalls and recommendations., PMID:36215988
Characterization of sabatolimab, a novel immunotherapy with immuno-myeloid activity directed against TIM-3 receptor., PMID:36196369
New Frontiers in Monoclonal Antibodies for the Targeted Therapy of Acute Myeloid Leukemia and Myelodysplastic Syndromes., PMID:35886899
Immune Checkpoint Inhibition in Acute Myeloid Leukemia and Myelodysplastic Syndromes., PMID:35883692
New Checkpoint Inhibitors on the Road: Targeting TIM-3 in Solid Tumors., PMID:35218498
Modulation of the Gal-9/TIM-3 Immune Checkpoint with α-Lactose. Does Anomery of Lactose Matter?, PMID:34944985
Advances in myelodysplastic syndrome., PMID:34474438
Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors., PMID:33883177
Management of patients with higher-risk myelodysplastic syndromes after failure of hypomethylating agents: What is on the horizon?, PMID:33762100