Catalog No.
DHC09607
Expression system
Mammalian Cells
Species reactivity
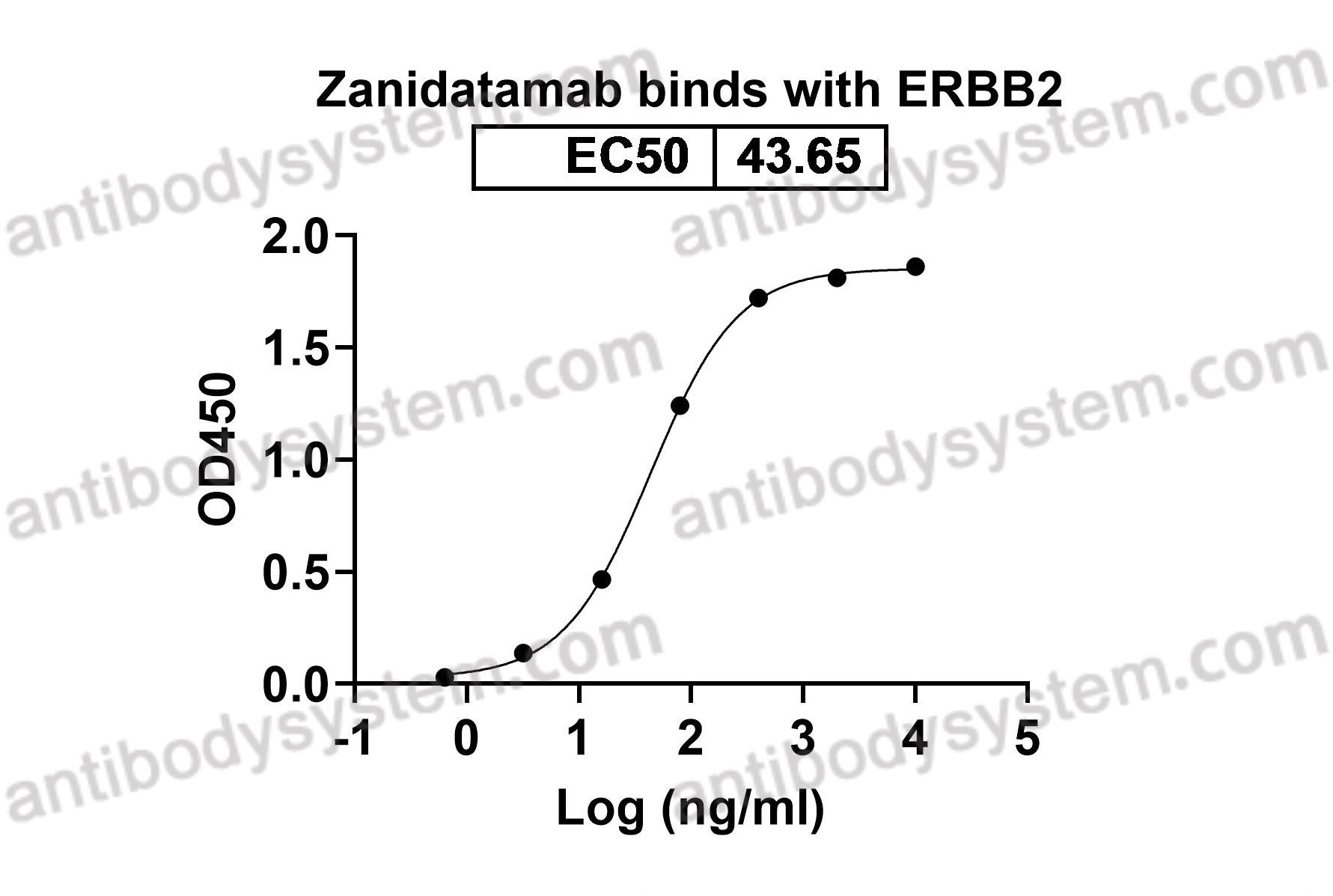
Human
Host species
Humanized
Isotype
IgG1-kappa/scFv-h-CH2-CH3
Clonality
Monoclonal
Target
p185erbB2, HER2, NGL, Tyrosine kinase-type cell surface receptor HER2, NEU, MLN 19, Proto-oncogene Neu, MLN19, ERBB2, Proto-oncogene c-ErbB-2, CD340, Receptor tyrosine-protein kinase erbB-2, Metastatic lymph node gene 19 protein
Concentration
1.46 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P04626
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
ZW-25, ZW25, zanidatamab zovodotin, CAS: 2169946-15-8
Clone ID
Zanidatamab
Zanidatamab, a bispecific monoclonal antibody for HER2-positive gastro-oesophageal adenocarcinoma patients: a new hope?, PMID:40473446
Zanidatamab plus chemotherapy as first-line treatment for patients with HER2-positive advanced gastro-oesophageal adenocarcinoma: primary results of a multicentre, single-arm, phase 2 study., PMID:40473445
Zanidatamab monotherapy or combined with chemotherapy in HER2-expressing gastroesophageal adenocarcinoma: a phase 1 trial., PMID:40341383
Zanidatamab plus palbociclib and fulvestrant in previously treated patients with hormone receptor-positive, HER2-positive metastatic breast cancer: primary results from a two-part, multicentre, single-arm, phase 2a study., PMID:40339592
Real-world efficacy of zanidatamab in patients with HER2 positive advanced biliary tract cancers., PMID:40319675
Safety and Efficacy of Anti-Human Epidermal Growth Factor 2 Agents in the Treatment of Biliary Tract Cancers: A Systematic Review., PMID:40239136
Zanidatamab: First Approval., PMID:40108069
Exploring Zanidatamab's efficacy across HER2-positive Malignancies: a narrative review., PMID:40025472
Novel drugs approved by the EMA, the FDA and the MHRA in 2024: A year in review., PMID:39971274
Zanidatamab (Ziihera) for biliary tract cancer., PMID:39819991
Antibodies to watch in 2025., PMID:39711140
[What's new in gastric cancer?]., PMID:39146748
Advances in HER2-Targeted Therapies: From monoclonal antibodies to dual inhibitors developments in cancer treatment., PMID:39137598
A plain language summary of the results from the phase 2b HERIZON-BTC-01 study of zanidatamab in participants with HER2-amplified biliary tract cancer., PMID:39114870
State of the art and upcoming trends in HER2-directed therapies in gastrointestinal malignancies., PMID:38726843
Co-clinical Trial of Novel Bispecific Anti-HER2 Antibody Zanidatamab in Patient-Derived Xenografts., PMID:38358339
Revolutionizing anti-HER2 therapies for extrahepatic cholangiocarcinoma and gallbladder cancer: Current advancements and future perspectives., PMID:38266541
A phase 2 trial of zanidatamab in HER2-overexpressed advanced endometrial carcinoma and carcinosarcoma (ZW25-IST-2)., PMID:38262242
New developments and standard of care in the management of advanced gastric cancer., PMID:37952913
Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study., PMID:37276871
An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity., PMID:36914633
Antibodies to watch in 2023., PMID:36472472
Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study., PMID:36400106
Population pharmacokinetics of zanidatamab, an anti-HER2 biparatopic antibody, in patients with advanced or metastatic cancer., PMID:36102999
HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma., PMID:36000541
Current and emerging therapies for advanced biliary tract cancers., PMID:34626563