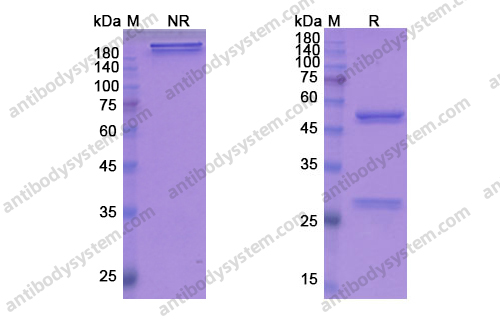
Catalog No.
DHJ53501
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-lambda
Clonality
Monoclonal
Target
Interleukin-22, Cytokine Zcyto18, IL-22, ZCYTO18, IL22, ILTIF, IL-TIF, IL-10-related T-cell-derived-inducible factor
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q9GZX6
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
ILV-094, CAS: 1007106-86-6
Clone ID
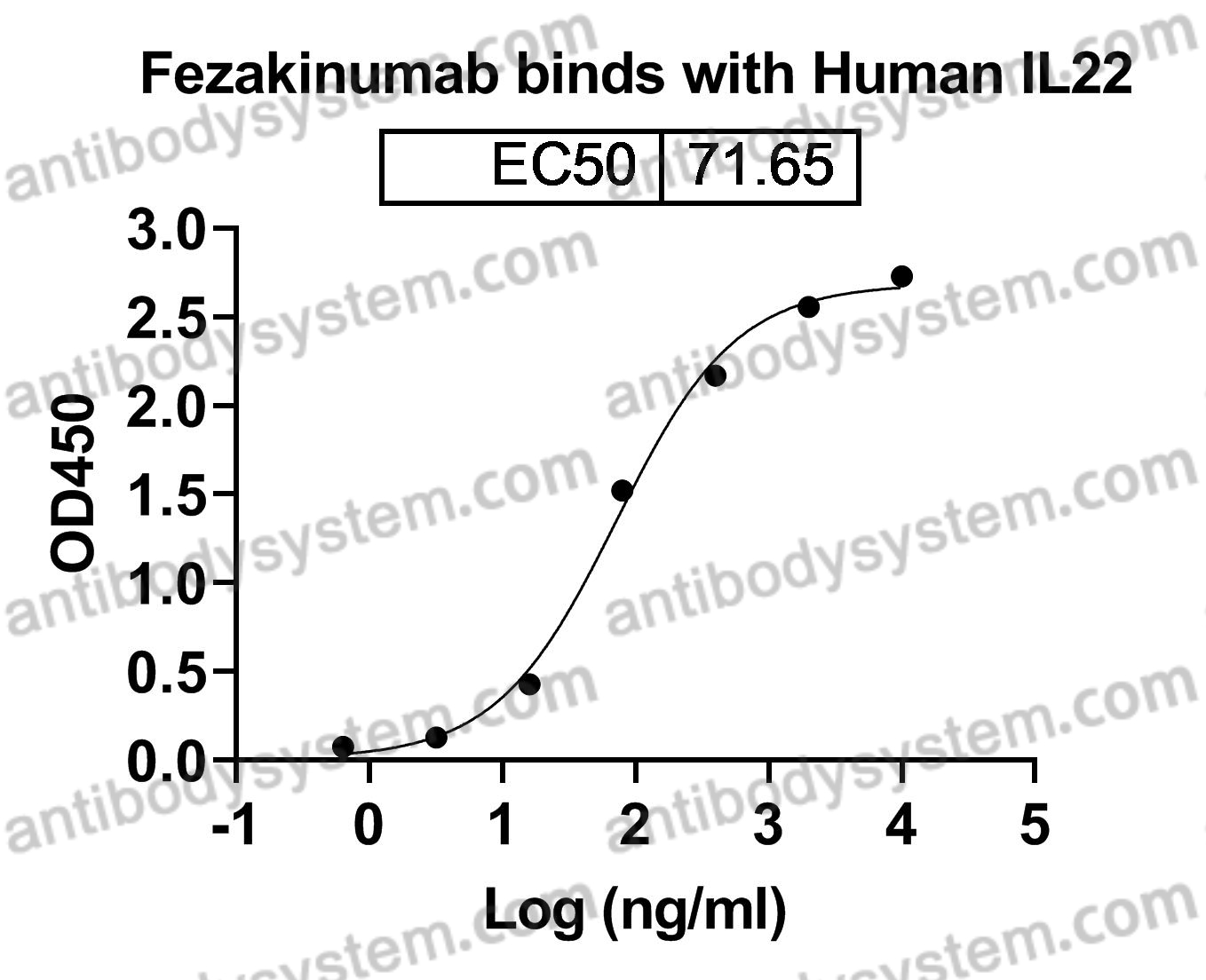
Fezakinumab
A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration, PMID: 33042126
A mathematical model to identify optimal combinations of drug targets for dupilumab poor responders in atopic dermatitis, PMID: 33894014
Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab, PMID: 30121291
Biological therapies for atopic dermatitis: An update, PMID: 30679974
Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial, PMID: 29353025
Efficacy of biologics in atopic dermatitis, PMID: 32003247
Mapping atopic dermatitis and anti-IL-22 response signatures to type 2-low severe neutrophilic asthma, PMID: 33891981
New therapies for atopic dermatitis: Additional treatment classes, PMID: 29248520
Novel systemic drugs in treatment of atopic dermatitis: results from phase II and phase III studies published in 2017/2018, PMID: 30095475
Novel Targeted Biological Agents for the Treatment of Atopic Dermatitis, PMID: 34213742
Novel Therapeutic Approaches and Targets for the Treatment of Atopic Dermatitis, PMID: 32525769
Review and analysis of biologic therapies currently in phase II and phase III clinical trials for atopic dermatitis, PMID: 32507066
Systemic Therapy of Atopic Dermatitis: Welcome to the Revolution, PMID: 29659645
The IL-20 Cytokine Family in Rheumatoid Arthritis and Spondyloarthritis, PMID: 30319661
What's New in Atopic Dermatitis, PMID: 30850043
IL-22 in Atopic Dermatitis., PMID:39195286
Interplay of cytokines in the pathophysiology of atopic dermatitis: insights from Murin models and human., PMID:38590314
Machine learning-based prediction models for atopic dermatitis diagnosis and evaluation., PMID:40528978
Safety and Danger Considerations of Novel Treatments for Atopic Dermatitis in Context of Primary Cutaneous Lymphomas., PMID:34948183
[Novel therapies in the treatment of atopic dermatitis]., PMID:34919093
Transcriptomic drug-response gene signatures are informative for the stratification of patients for clinical trials., PMID:34582880
Novel Targeted Biological Agents for the Treatment of Atopic Dermatitis., PMID:34213742
A mathematical model to identify optimal combinations of drug targets for dupilumab poor responders in atopic dermatitis., PMID:33894014
Mapping atopic dermatitis and anti-IL-22 response signatures to type 2-low severe neutrophilic asthma., PMID:33891981
Biological Therapies for Atopic Dermatitis: A Systematic Review., PMID:33735876
A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration., PMID:33042126
Novel Therapeutic Approaches and Targets for the Treatment of Atopic Dermatitis., PMID:32525769
Review and analysis of biologic therapies currently in phase II and phase III clinical trials for atopic dermatitis., PMID:32507066
Efficacy of biologics in atopic dermatitis., PMID:32003247
What's New in Atopic Dermatitis., PMID:30850043
Biological therapies for atopic dermatitis: An update., PMID:30679974
The IL-20 Cytokine Family in Rheumatoid Arthritis and Spondyloarthritis., PMID:30319661
Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab., PMID:30121291
Novel systemic drugs in treatment of atopic dermatitis: results from phase II and phase III studies published in 2017/2018., PMID:30095475
Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial., PMID:29353025
New therapies for atopic dermatitis: Additional treatment classes., PMID:29248520
Systemic Therapy of Atopic Dermatitis: Welcome to the Revolution., PMID:29659645