Catalog No.
DHJ70110
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG4-lambda
Clonality
Monoclonal
Target
B7-H1, Programmed cell death 1 ligand 1, PDCD1 ligand 1, PDCD1L1, B7 homolog 1, PDCD1LG1, PDL1, hPD-L1, Programmed death ligand 1, B7H1, PD-L1, CD274
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
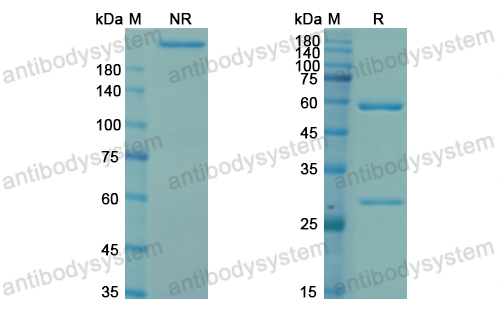
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q9NZQ7
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Clone ID
Sugemalimab
Comparative outcomes of first-line PD-1/PD-L1 inhibitors plus chemotherapy for advanced squamous non-small cell lung cancer: a systematic review and network meta-analysis of randomized clinical trials., PMID:40114962
First-Line Sugemalimab Plus Chemotherapy for Advanced Gastric Cancer: The GEMSTONE-303 Randomized Clinical Trial., PMID:39992668
An evaluation of sugemalimab for the treatment of relapsed or refractory extranodal natural killer T-cell lymphoma., PMID:39702924
Comprehensive population pharmacokinetic modelling of sugemalimab, an anti-programmed death-ligand 1 (PD-L1) human monoclonal antibody, in patients with solid tumours or lymphomas across multiple Phase I-III studies., PMID:39389094
Sugemalimab combined with chemotherapy for the treatment of advanced esophageal squamous cell carcinoma: a cost-effectiveness analysis., PMID:39005941
Clinical Efficacy of Programmed Cell Death Ligand 1 Antibody in Treatment of Extranodal Natural Killer/T-Cell Lymphoma With Hemophagocytic Lymphohistiocytosis., PMID:38644986
Cost-effectiveness of immunotherapies for advanced squamous non-small cell lung cancer: a systematic review., PMID:38448878
Immune checkpoint inhibitors or anti-claudin 18.2 antibodies? A network meta-analysis for the optimized first-line therapy of HER2-negative gastric cancer., PMID:38362378
Sugemalimab with chemotherapy for advanced ESCC., PMID:38355754
Clinical benefit of anti-PD-1/PD-L1 plus chemotherapy in first-line treatment for patients over the age of 65 or 75 with metastatic non-small cell lung cancer (NSCLC)., PMID:38303601
First-line sugemalimab with chemotherapy for advanced esophageal squamous cell carcinoma: a randomized phase 3 study., PMID:38302715
Advances and challenges of first-line immunotherapy for non-small cell lung cancer: A review., PMID:38241591
Author Correction: Interim survival analysis of the randomized phase III GEMSTONE-302 trial: sugemalimab or placebo plus chemotherapy as first-line treatment for metastatic NSCLC., PMID:38182915
Tumor growth inhibition-overall survival modeling in non-small cell lung cancer: A case study from GEMSTONE-302., PMID:38111189
Encouraging prospects with sugemalimab in relapsed or refractory extranodal natural killer/T-cell lymphoma., PMID:38073311
Comparison of the profiles of first-line PD-1/PD-L1 inhibitors for advanced NSCLC lacking driver gene mutations: a systematic review and Bayesian network meta-analysis., PMID:37841212
Case Report: Replacement of PD-1 inhibitors with PD-L1 inhibitors in the treatment of squamous non-small-cell lung carcinoma., PMID:37649479
Transformation of Lung Squamous Cell Carcinoma to Small Cell Lung Cancer After Immunotherapy Resistance: A Case Report., PMID:37583652
The Cost-Effectiveness of Sugemalimab Plus Chemotherapy as First-Line Treatment for Metastatic Squamous and Non-squamous NSCLC in China., PMID:37453992
Design and Rationale for a Phase II/III, Randomized, Double-Blind, Placebo-Controlled Study of Sugemalimab as Consolidation Therapy in Patients With Limited-Stage Small-Cell Lung Cancer Who Have Not Progressed Following Concurrent or Sequential Chemoradiotherapy: The SURPASS Study., PMID:37442748
Interim survival analysis of the randomized phase III GEMSTONE-302 trial: sugemalimab or placebo plus chemotherapy as first-line treatment for metastatic NSCLC., PMID:37322367
A trial-based cost-utility analysis of sugemalimab vs. placebo as consolidation therapy for unresectable stage III NSCLC in China., PMID:37262075
Economic evaluation of five first-line PD-(L)1 inhibitors for treating non-squamous non-small cell lung cancer in China: A cost-effectiveness analysis based on network meta-analysis., PMID:37021058
Sugemalimab Monotherapy for Patients With Relapsed or Refractory Extranodal Natural Killer/T-Cell Lymphoma (GEMSTONE-201): Results From a Single-Arm, Multicenter, Phase II Study., PMID:36996373
Sugemalimab plus chemotherapy vs. chemotherapy for metastatic non-small-cell lung cancer: A cost-effectiveness analysis., PMID:36923040
Effectiveness and cost-effectiveness analysis of 11 treatment paths, seven first-line and three second-line treatments for Chinese patients with advanced wild-type squamous non-small cell lung cancer: A sequential model., PMID:36908446
Sugemalimab, a novel PD-L1 inhibitor for treatment of advanced or metastatic non-small cell lung cancer., PMID:36847625
The safety of combining immune checkpoint inhibitors and platinum-based chemotherapy for the treatment of solid tumors: A systematic review and network meta-analysis., PMID:36825025
Economic evaluation of first-line sugemalimab plus chemotherapy for metastatic non-small cell lung cancer in China., PMID:36582798
Cost-effectiveness analysis of sugemalimab in combination with chemotherapy as first-line treatment in Chinese patients with metastatic NSCLC., PMID:36413882
GEMSTONE-302: "If you keep doing the same thing you get the same result"., PMID:36408544
Cost-effectiveness analysis of sugemalimab vs. placebo, in combination with chemotherapy, for treatment of first-line metastatic NSCLC in China., PMID:36408023
Identifying optimal PD-1/PD-L1 inhibitors in first-line treatment of patients with advanced squamous non-small cell lung cancer in China: Updated systematic review and network meta-analysis., PMID:36249794
Landscape of the clinical development of China innovative anti-lung cancer drugs., PMID:38328605
Cost-effectiveness analysis of sugemalimab vs. chemotherapy as first-line treatment of metastatic nonsquamous non-small cell lung cancer., PMID:36172187
Sequential chemoradiotherapy followed by sugemalimab for locally advanced NSCLC - Author's reply., PMID:35358459
Sequential chemoradiotherapy followed by sugemalimab for locally advanced NSCLC., PMID:35358458
Sugemalimab: First Approval., PMID:35298827
Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial., PMID:35038432
Promising outlook with sugemalimab in non-small-cell lung cancer., PMID:35038431
Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial., PMID:35038429
Safety, antitumor activity and biomarkers of sugemalimab in Chinese patients with advanced solid tumors or lymphomas: results from the first-in-human phase 1 trial., PMID:34984540