Catalog No.
DHJ70108
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
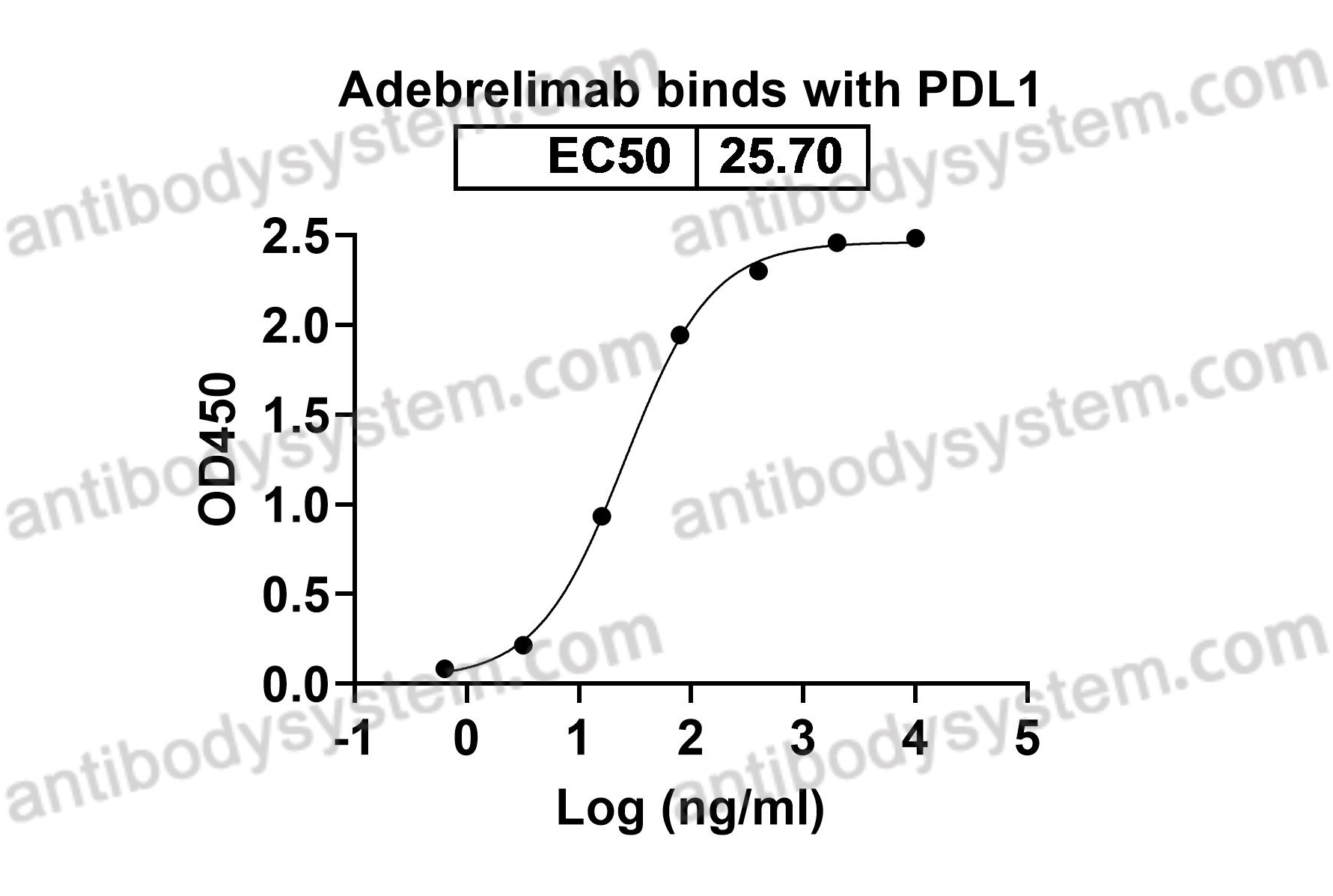
B7-H1, Programmed cell death 1 ligand 1, PDCD1 ligand 1, PDCD1L1, B7 homolog 1, PDCD1LG1, PDL1, hPD-L1, Programmed death ligand 1, B7H1, PD-L1, CD274
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
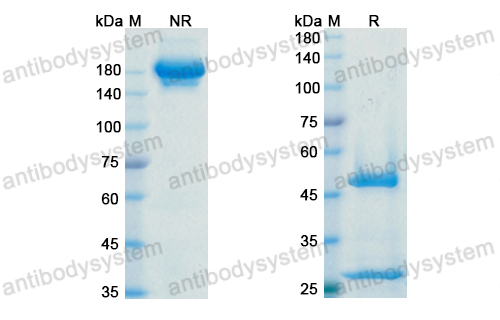
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q9NZQ7
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
HTI-1088, SHR-1316, CAS: 2247114-85-6
Clone ID
Adebrelimab
Cost-effectiveness analysis of adebrelimab in combination with chemotherapy for first-line treatment of extensive-stage small cell lung cancer., PMID:40512778
Adebrelimab in Small Cell Lung Cancer: From Current Advances to Emerging Combination Strategy and Challenge., PMID:40475251
Clinical study of adebrelimab in combination with apatinib and irinotecan for PD-1 inhibitor-ineffective advanced-stage gastric cancer: study protocol for a single-arm, single-centre, exploratory trial., PMID:40467306
Effects of perioperative treatment of resectable adenocarcinoma of esophagogastric junction by immunotherapy (Adebrelimab) combined with chemotherapy (XELOX): protocol for a single-center, open-labeled study (AEGIS trial, neoadjuvant immunochemotherapy)., PMID:39905364
Phase I Clinical Trial of Autologous Hematopoietic Stem Cell Transplantation-Supported Dose-Intensified Chemotherapy With Adebrelimab as First-Line Treatment for Extensive-Stage Small Cell Lung Cancer., PMID:39848827
Neoadjuvant adebrelimab combined with chemotherapy (cisplatin/carboplatin and etoposide) for limited-stage small-cell lung cancer: a study protocol of phase 2 trial (NIUS)., PMID:39800398
A prospective, phase II, neoadjuvant study based on chemotherapy sensitivity in HR+/HER2- breast cancer-FINEST study., PMID:39754712
[18F]FDG PET/CT for predicting neoadjuvant PD-L1 blockade monotherapy treatment response in patients with locally advanced esophageal squamous cell carcinoma: a preliminary study., PMID:39743618
Progress of research on PD-L1 inhibitor adebrelimab usage in malignant tumors., PMID:39687879
Adebrelimab versus serplulimab plus chemotherapy as first-line treatment for extensive-stage small-cell lung cancer: A cost-effectiveness analysis in China., PMID:39617637
Case report: Immune checkpoint inhibitor-induced paraneoplastic neurological syndrome in two patients: a case series., PMID:39529834
SHR-8068 combined with adebrelimab and bevacizumab in the treatment of refractory advanced colorectal cancer: study protocol for a single-arm, phase Ib/II study., PMID:39445023
Adebrelimab plus chemotherapy and sequential thoracic radiotherapy as first-line therapy for extensive-stage small-cell lung cancer (ES-SCLC): a phase II trial., PMID:39252865
Potential therapeutic option for EGFR-mutant small cell lung cancer transformation: a case report and literature review., PMID:39234244
First-line treatments for extensive-stage small-cell lung cancer with immune checkpoint inhibitors plus chemotherapy: a China-based cost-effectiveness analysis., PMID:39035009
Population pharmacokinetics of adebrelimab - Support of alternative flat dose regimen in extensive-stage small-cell lung cancer., PMID:38711252
Efficacy and safety of novel immune checkpoint inhibitor-based combinations versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer: A network meta-analysis., PMID:38623838
Updated cost-effectiveness analysis of adebrelimab plus chemotherapy for extensive-stage small cell lung cancer in China., PMID:38582540
Case report: A novel perspective on the treatment of primary tracheal small cell carcinoma: a patient's experience with immuno-combined EP therapy and literature review., PMID:38348051
Antibodies to watch in 2024., PMID:38178784
Effects of neoadjuvant stereotactic body radiotherapy plus adebrelimab and chemotherapy for triple-negative breast cancer: A pilot study., PMID:38131294
Drug Pricing of Domestic Anti-PD-L1 Antibody Adebrelimab: Cost-Effectiveness Analysis of the First-Line ES-SCLC Treatment in China., PMID:38024490
Comparative efficacy and safety of novel immuno-chemotherapy for extensive-stage small-cell lung cancer: a network meta-analysis of randomized controlled trial., PMID:37846397
Adebrelimab plus chemotherapy vs. chemotherapy for treatment of extensive-stage small-cell lung cancer from the US and Chinese healthcare sector perspectives: a cost-effectiveness analysis to inform drug pricing., PMID:37547339
Publisher Correction: Neoadjuvant adebrelimab in locally advanced resectable esophageal squamous cell carcinoma: a phase 1b trial., PMID:37507607
Neoadjuvant adebrelimab in locally advanced resectable esophageal squamous cell carcinoma: a phase 1b trial., PMID:37488287
Efficacy and safety of first-line immunotherapy plus chemotherapy in treating patients with extensive-stage small cell lung cancer: a Bayesian network meta-analysis., PMID:37435087
Cost-effectiveness of the combination of immunotherapy and chemotherapy for extensive-stage small-cell lung cancer: a systematic review., PMID:37365540
The efficacy of adebrelimab compared with durvalumab and atezolizumab in untreated extensive-stage small-cell lung cancer: a survival analysis of reconstructed patient-level data., PMID:37215120
Efficacy and safety of immune checkpoint inhibitors combined with chemotherapy in patients with extensive-stage small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials., PMID:37152041
Effectiveness and safety of first-line immune checkpoint inhibitors for patients with extensive-stage small cell lung carcinoma: A systematic review and network meta-analysis., PMID:37095958
Efficacy and safety of first-line immune checkpoint inhibitors combined with chemotherapy for extensive-stage small cell lung cancer: A network meta-analysis., PMID:36774774
Cost-effectiveness analysis of adebrelimab combined with chemotherapy for extensive-stage small cell lung cancer., PMID:36386191
Adebrelimab (SHR-1316) in Combination With Chemotherapy as Perioperative Treatment in Patients With Resectable Stage II to III NSCLCs: An Open-Label, Multicenter, Phase 1b Trial., PMID:36191882
Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial., PMID:35576956