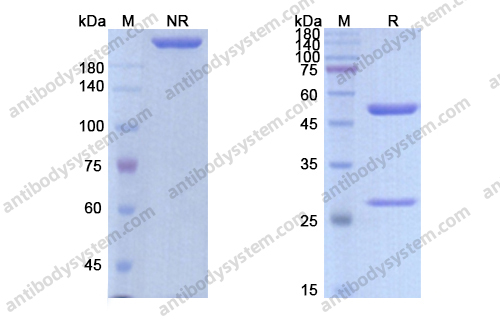
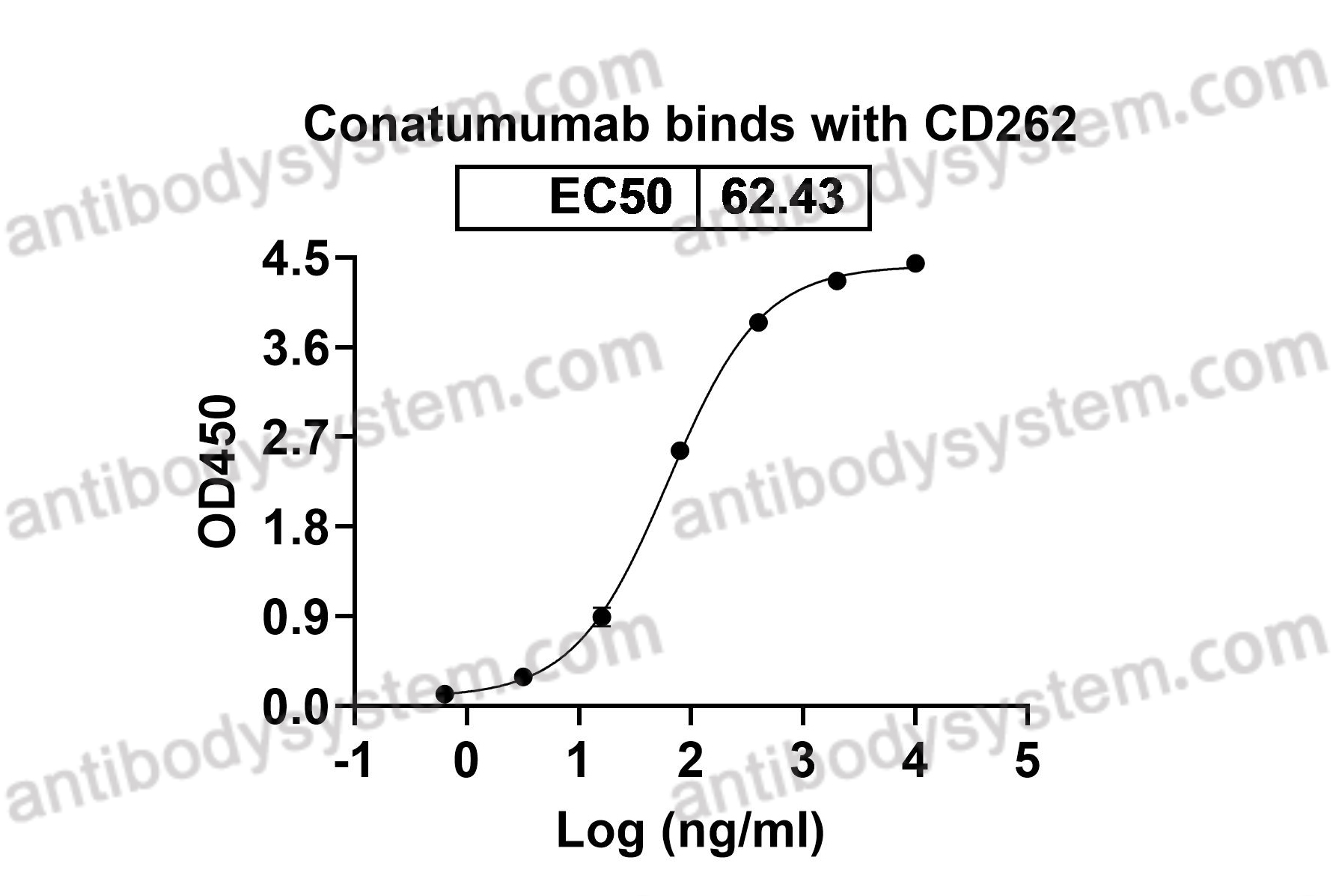
Conatumumab, a fully human mAb against death receptor 5 for the treatment of cancer, PMID: 20496264
Conatumumab: a novel monoclonal antibody against death receptor 5 for the treatment of advanced malignancies in adults, PMID: 21877997
64 Cu-Tetraazacyclododecane- N, N', N'', N'''-tetraacetic acid-conatumumab, PMID: 22220318
Characterization of 64Cu-DOTA-conatumumab: a PET tracer for in vivo imaging of death receptor 5, PMID: 21571804
Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types, PMID: 20150762
Conatumumab (AMG 655) coated nanoparticles for targeted pro-apoptotic drug delivery, PMID: 21875750
A first-in-human study of conatumumab in adult patients with advanced solid tumors, PMID: 20947515
Durable Complete Response to AMG 655 (Conatumumab) and Vorinostat in a Patient With Relapsed Classical Hodgkin Lymphoma: Extraordinary Response from a Phase 1b Clinical Protocol, PMID: 32828719
Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors, PMID: 21161528
TRAIL receptor agonist conatumumab with modified FOLFOX6 plus bevacizumab for first-line treatment of metastatic colorectal cancer: A randomized phase 1b/2 trial, PMID: 24122767
First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: a phase I/II open-label and double-blind study, PMID: 22240283
Measurement of conatumumab-induced apoptotic activity in tumors by fine needle aspirate sampling, PMID: 20623688
A randomized phase 2 study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer, PMID: 23370314
A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer, PMID: 22700995
Anticancer activity of the type I insulin-like growth factor receptor antagonist, ganitumab, in combination with the death receptor 5 agonist, conatumumab, PMID: 24816908
A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer, PMID: 23510984
Efficacy and Safety of Regorafenib in Combination with Chemotherapy as Second-Line Treatment in Patients with Metastatic Colorectal Cancer: A Network Meta-Analysis and Systematic Literature Review, PMID: 32770529
The serum protein transthyretin as a platform for dimerization and tetramerization of antibodies and Fab fragments to enable target clustering, PMID: 32518163
Caspase-8 expression is predictive of tumour response to death receptor 5 agonist antibody in Ewing's sarcoma, PMID: 26291055
Onto better TRAILs for cancer treatment, PMID: 26943322
Intracellular-signaling tumor-regression modeling of the pro-apoptotic receptor agonists dulanermin and conatumumab, PMID: 22932917
Death receptors as targets in cancer, PMID: 23638798
A network meta-analysis on the efficacy of sixteen targeted drugs in combination with chemotherapy for treatment of advanced/metastatic colorectal cancer, PMID: 27806321
99m Tc-Duramycin SPECT Imaging of Early Tumor Response to Targeted Therapy: A Comparison with 18 F-FDG PET, PMID: 27879368
Meta-analysis of first-line therapies with maintenance regimens for advanced non-small-cell lung cancer (NSCLC) in molecularly and clinically selected populations, PMID: 28675660
Therapeutic targeting of the TNF superfamily: a promising treatment for advanced endometrial adenocarcinoma, PMID: 22885470
Gateways to clinical trials, PMID: 19798455
[ 99m Tc]duramycin for cell death imaging: Impact of kit formulation, purification and species difference, PMID: 29031229
TRAIL-R2-specific antibodies and recombinant TRAIL can synergise to kill cancer cells, PMID: 24909167
Implementation of design of experiments (DOE) in the development and validation of a cell-based bioassay for the detection of anti-drug neutralizing antibodies in human serum, PMID: 22119514
Validity of the FACT Hepatobiliary (FACT-Hep) questionnaire for assessing disease-related symptoms and health-related quality of life in patients with metastatic pancreatic cancer, PMID: 22678353
Apo2L/TRAIL and the death receptor 5 agonist antibody AMG 655 cooperate to promote receptor clustering and antitumor activity, PMID: 25043603
Benzazepinones and benzoxazepinones as antagonists of inhibitor of apoptosis proteins (IAPs) selective for the second baculovirus IAP repeat (BIR2) domain, PMID: 24083782
American Society of Hematology--51st annual meeting & exposition. Part 1, PMID: 20127549
Leveraging image cytometry for the development of clinically feasible biomarkers: evaluation of activated caspase-3 in fine needle aspirate biopsies, PMID: 21704844
The generation of stable microvessels in ischemia is mediated by endothelial cell derived TRAIL., PMID:39365855
TRAIL-Based Therapies Efficacy in Pediatric Bone Tumors Models Is Modulated by TRAIL Non-Apoptotic Pathway Activation via RIPK1 Recruitment., PMID:36428719
Combined and targeted drugs delivery system for colorectal cancer treatment: Conatumumab decorated, reactive oxygen species sensitive irinotecan prodrug and quercetin co-loaded nanostructured lipid carriers., PMID:35049388
Durable Complete Response to AMG 655 (Conatumumab) and Vorinostat in a Patient With Relapsed Classical Hodgkin Lymphoma: Extraordinary Response from a Phase 1b Clinical Protocol., PMID:32828719
Efficacy and Safety of Regorafenib in Combination with Chemotherapy as Second-Line Treatment in Patients with Metastatic Colorectal Cancer: A Network Meta-Analysis and Systematic Literature Review., PMID:32770529
The serum protein transthyretin as a platform for dimerization and tetramerization of antibodies and Fab fragments to enable target clustering., PMID:32518163
[99mTc]duramycin for cell death imaging: Impact of kit formulation, purification and species difference., PMID:29031229
Meta-analysis of first-line therapies with maintenance regimens for advanced non-small-cell lung cancer (NSCLC) in molecularly and clinically selected populations., PMID:28675660
99mTc-Duramycin SPECT Imaging of Early Tumor Response to Targeted Therapy: A Comparison with 18F-FDG PET., PMID:27879368
A network meta-analysis on the efficacy of sixteen targeted drugs in combination with chemotherapy for treatment of advanced/metastatic colorectal cancer., PMID:27806321
Onto better TRAILs for cancer treatment., PMID:26943322
Caspase-8 expression is predictive of tumour response to death receptor 5 agonist antibody in Ewing's sarcoma., PMID:26291055
Apo2L/TRAIL and the death receptor 5 agonist antibody AMG 655 cooperate to promote receptor clustering and antitumor activity., PMID:25043603
TRAIL-R2-specific antibodies and recombinant TRAIL can synergise to kill cancer cells., PMID:24909167
Anticancer activity of the type I insulin-like growth factor receptor antagonist, ganitumab, in combination with the death receptor 5 agonist, conatumumab., PMID:24816908
TRAIL receptor agonist conatumumab with modified FOLFOX6 plus bevacizumab for first-line treatment of metastatic colorectal cancer: A randomized phase 1b/2 trial., PMID:24122767
Benzazepinones and benzoxazepinones as antagonists of inhibitor of apoptosis proteins (IAPs) selective for the second baculovirus IAP repeat (BIR2) domain., PMID:24083782
Death receptors as targets in cancer., PMID:23638798
A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer., PMID:23510984
A randomized phase 2 study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer., PMID:23370314
Intracellular-signaling tumor-regression modeling of the pro-apoptotic receptor agonists dulanermin and conatumumab., PMID:22932917
Therapeutic targeting of the TNF superfamily: a promising treatment for advanced endometrial adenocarcinoma., PMID:22885470
A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer., PMID:22700995
Validity of the FACT Hepatobiliary (FACT-Hep) questionnaire for assessing disease-related symptoms and health-related quality of life in patients with metastatic pancreatic cancer., PMID:22678353
First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: a phase I/II open-label and double-blind study., PMID:22240283
Implementation of design of experiments (DOE) in the development and validation of a cell-based bioassay for the detection of anti-drug neutralizing antibodies in human serum., PMID:22119514
Conatumumab: a novel monoclonal antibody against death receptor 5 for the treatment of advanced malignancies in adults., PMID:21877997
Conatumumab (AMG 655) coated nanoparticles for targeted pro-apoptotic drug delivery., PMID:21875750
Leveraging image cytometry for the development of clinically feasible biomarkers: evaluation of activated caspase-3 in fine needle aspirate biopsies., PMID:21704844
Characterization of 64Cu-DOTA-conatumumab: a PET tracer for in vivo imaging of death receptor 5., PMID:21571804
Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors., PMID:21161528
A first-in-human study of conatumumab in adult patients with advanced solid tumors., PMID:20947515
Measurement of conatumumab-induced apoptotic activity in tumors by fine needle aspirate sampling., PMID:20623688
Conatumumab, a fully human mAb against death receptor 5 for the treatment of cancer., PMID:20496264
Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types., PMID:20150762
American Society of Hematology--51st annual meeting & exposition. Part 1., PMID:20127549
Gateways to clinical trials., PMID:19798455