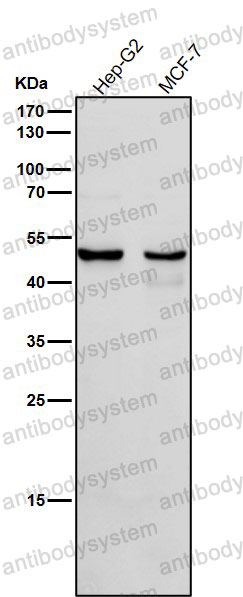
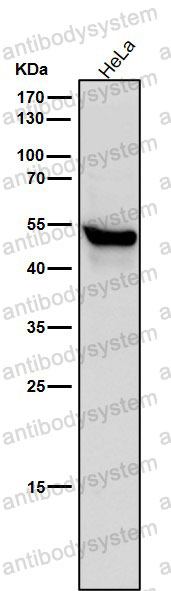
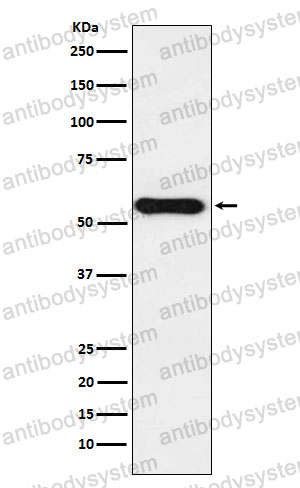
Catalog No.
RHC15101
Species reactivity
Human
Host species
Rabbit
Isotype
IgG
Clonality
Monoclonal
Tested applications
FCM: 1:20-1:100, IF: 1:50-1:200, WB: 1:1000-1:2000
Target
Hydroperoxy icosatetraenoate dehydratase, Cytochrome P450-P3, Cytochrome P450 4, CYP1A2, Cholesterol 25-hydroxylase, CYPIA2, Cytochrome P(3)450, Cytochrome P450 1A2
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P05177
Applications
FCM, IF, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 0.05% BSA, 50% Glycerol, 0.05% Sodium azide.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 4 ℃ for frequent use. Store at -20 ℃ for twelve months from the date of receipt.
Clone ID
R1K15
Evaluation of the Effect of Risankizumab on the Pharmacokinetics of Cytochrome P450 Substrates in Patients with Moderately to Severely Active Ulcerative Colitis or Crohn's Disease., PMID:39707077
Evaluation of Drug-Drug Interaction Potential of Talquetamab, a T-Cell-Redirecting GPRC5D × CD3 Bispecific Antibody, as a Result of Cytokine Release Syndrome in Patients with Relapsed/Refractory Multiple Myeloma in MonumenTAL-1, Using a Physiologically Based Pharmacokinetic Model., PMID:39285155
Inflammation-mediated drug interactions of olokizumab and cytochrome P450 activities in patients with rheumatoid arthritis., PMID:38984761
In Vitro Metabolism and Transport Characteristics of Zastaprazan., PMID:38931920
Evaluation of the potential impact on pharmacokinetics of various cytochrome P450 substrates of increasing IL-6 levels following administration of the T-cell bispecific engager glofitamab., PMID:38044486
Lactobacillus species ameliorate membranous nephropathy through inhibiting the aryl hydrocarbon receptor pathway via tryptophan-produced indole metabolites., PMID:37594378
The Influence of Lipid Microdomain Heterogeneity on Protein-Protein Interactions: Proteomic Analysis of Co-Immunoprecipitated Binding Partners of P450 1A2 and P450 3A in Rat Liver Microsomes., PMID:37349115
The multikinase inhibitor axitinib in the treatment of advanced hepatocellular carcinoma: the current clinical applications and the molecular mechanisms., PMID:37325670
Liver microsomal cytochrome P450 3A-dependent drug oxidation activities in individual dogs., PMID:37144920
Impairment of aryl hydrocarbon receptor signalling promotes hepatic disorders in cancer cachexia., PMID:37127348
Focal adhesion alterations in G0-positive melanoma cells., PMID:36533319
Adenosine N6-methylation upregulates the expression of human CYP2B6 by altering the chromatin status., PMID:36113565
The Effect of Triptolide Combined With Crocin on Arthritis in Mice: From Side Effect Attenuation to Therapy., PMID:35814255
Acute tubulointerstitial nephritis associated with antineutrophil cytoplasmic antibody following cimetidine treatment: a case report., PMID:34461843
Improving Antibody Production in Stably Transfected CHO Cells by CRISPR-Cas9-Mediated Inactivation of Genes Identified in a Large-Scale Screen with Chinese Hamster-Specific siRNAs., PMID:33079482
Evaluation of Montelukast for the Prevention of Infusion-related Reactions With Daratumumab., PMID:32660902
What's new in atopic eczema? An analysis of systematic reviews published in 2018. Part 2: systemic therapies., PMID:32568435
Evaluating Potential Disease-Mediated Protein-Drug Interactions in Patients With Moderate-to-Severe Plaque Psoriasis Receiving Subcutaneous Guselkumab., PMID:32407591
Enrichment-free High-throughput Liquid Chromatography-Multiple-Reaction Monitoring Quantification of Cytochrome P450 Proteins in Plated Human Hepatocytes Direct from 96-Well Plates Enables Routine Protein Induction Measurements., PMID:32350061
Mertansine Inhibits mRNA Expression and Enzyme Activities of Cytochrome P450s and Uridine 5'-Diphospho-Glucuronosyltransferases in Human Hepatocytes and Liver Microsomes., PMID:32131538
Methylation of adenosine at the N6 position post-transcriptionally regulates hepatic P450s expression., PMID:31706844
Effect of Dietary Doses of Quercetin on Hepatic Drug Metabolizing Enzymes in Spontaneously Hypertensive Rats., PMID:31065969
Physicochemical Properties, Biotransformation, and Transport Pathways of Established and Newly Approved Medications: A Systematic Review of the Top 200 Most Prescribed Drugs vs. the FDA-Approved Drugs Between 2005 and 2016., PMID:30972694
Lack of Effect of 12-Week Treatment with Risankizumab on the Pharmacokinetics of Cytochrome P450 Probe Substrates in Patients with Moderate to Severe Chronic Plaque Psoriasis., PMID:30574672
CYP2J2 is the major enzyme in human liver microsomes responsible for hydroxylation of SYL-927, a novel and selective sphingosine 1-phosphate receptor 1 (S1P1 ) agonist., PMID:30362120
Site-specific oxidation of flavanone and flavone by cytochrome P450 2A6 in human liver microsomes., PMID:30048196
Evaluation of Potential Disease-Mediated Drug-Drug Interaction in Patients With Moderate-to-Severe Atopic Dermatitis Receiving Dupilumab., PMID:29498038
CYP Suppression in Human Hepatocytes by Monomethyl Auristatin E, the Payload in Brentuximab Vedotin (Adcetris®), is Associated with Microtubule Disruption., PMID:29264831
The JAK1/2 Inhibitor Ruxolitinib Reverses Interleukin-6-Mediated Suppression of Drug-Detoxifying Proteins in Cultured Human Hepatocytes., PMID:29162613
Comparison of In Vitro Hepatic Scoparone 7-O-Demethylation between Humans and Experimental Animals., PMID:28950382
Concept: The Use of Targeted Immunoaffinity Proteomics for Routine Assessment of In Vitro Enzyme Induction., PMID:28778426
Levofloxacin-Induced Acute Immune-Mediated Thrombocytopenia of Rapid-Onset., PMID:28403679
Metabolic characterization of (1-(5-fluoropentyl)-1H-indol-3-yl)(4-methyl-1-naphthalenyl)-methanone (MAM-2201) using human liver microsomes and cDNA-overexpressed cytochrome P450 enzymes., PMID:27924364
Application of substrate depletion assay to evaluation of CYP isoforms responsible for stereoselective metabolism of carvedilol., PMID:27836712
Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior., PMID:27702941
Pharmacokinetics and Differential Regulation of Cytochrome P450 Enzymes in Type 1 Allergic Mice., PMID:27694226
Role of aryl hydrocarbon receptor polymorphisms on TCDD-mediated CYP1B1 induction and IgM suppression by human B cells., PMID:27535091
Therapeutic protein-drug interaction assessment for daclizumab high-yield process in patients with multiple sclerosis using a cocktail approach., PMID:26991517
Development of a Physiologically Based Pharmacokinetic Model to Predict Disease-Mediated Therapeutic Protein-Drug Interactions: Modulation of Multiple Cytochrome P450 Enzymes by Interleukin-6., PMID:26961818
Co-expression of active human cytochrome P450 1A2 and cytochrome P450 reductase on the cell surface of Escherichia coli., PMID:26838175
Physiologically Based Pharmacokinetic Model to Assess the Influence of Blinatumomab-Mediated Cytokine Elevations on Cytochrome P450 Enzyme Activity., PMID:26451330
CYP450 Enzyme-Mediated Metabolism of TCAS and Its Inhibitory and Induced Effects on Metabolized Enzymes in Vitro., PMID:26404338
Metabolism of (-)-cis- and (-)-trans-rose oxide by cytochrome P450 enzymes in human liver microsomes., PMID:26126958
Dietary Doses of Sulforaphane Affect Hepatic Drug Metabolizing Enzymes in Spontaneously Hypertensive Rats., PMID:26084424
Evaluation of disease-mediated therapeutic protein-drug interactions between an anti-interleukin-6 monoclonal antibody (sirukumab) and cytochrome P450 activities in a phase 1 study in patients with rheumatoid arthritis using a cocktail approach., PMID:26054042
Comprehensive kinetic analysis and influence of reaction components for chlorzoxazone 6-hydroxylation in human liver microsomes with CYP antibodies., PMID:25815637
Enzymatic characterization of in vitro-expressed Baikal seal cytochrome P450 (CYP) 1A1, 1A2, and 1B1: implication of low metabolic potential of CYP1A2 uniquely evolved in aquatic mammals., PMID:25814058
mRNA profiling reveals determinants of trastuzumab efficiency in HER2-positive breast cancer., PMID:25710561
Anti-CD28 monoclonal antibody-stimulated cytokines released from blood suppress CYP1A2, CYP2B6, and CYP3A4 in human hepatocytes in vitro., PMID:25326287
[DIO2, TPO, CYP1A1 AND CYP1A2 gene polymorphism in women with thyroid disease]., PMID:25306702