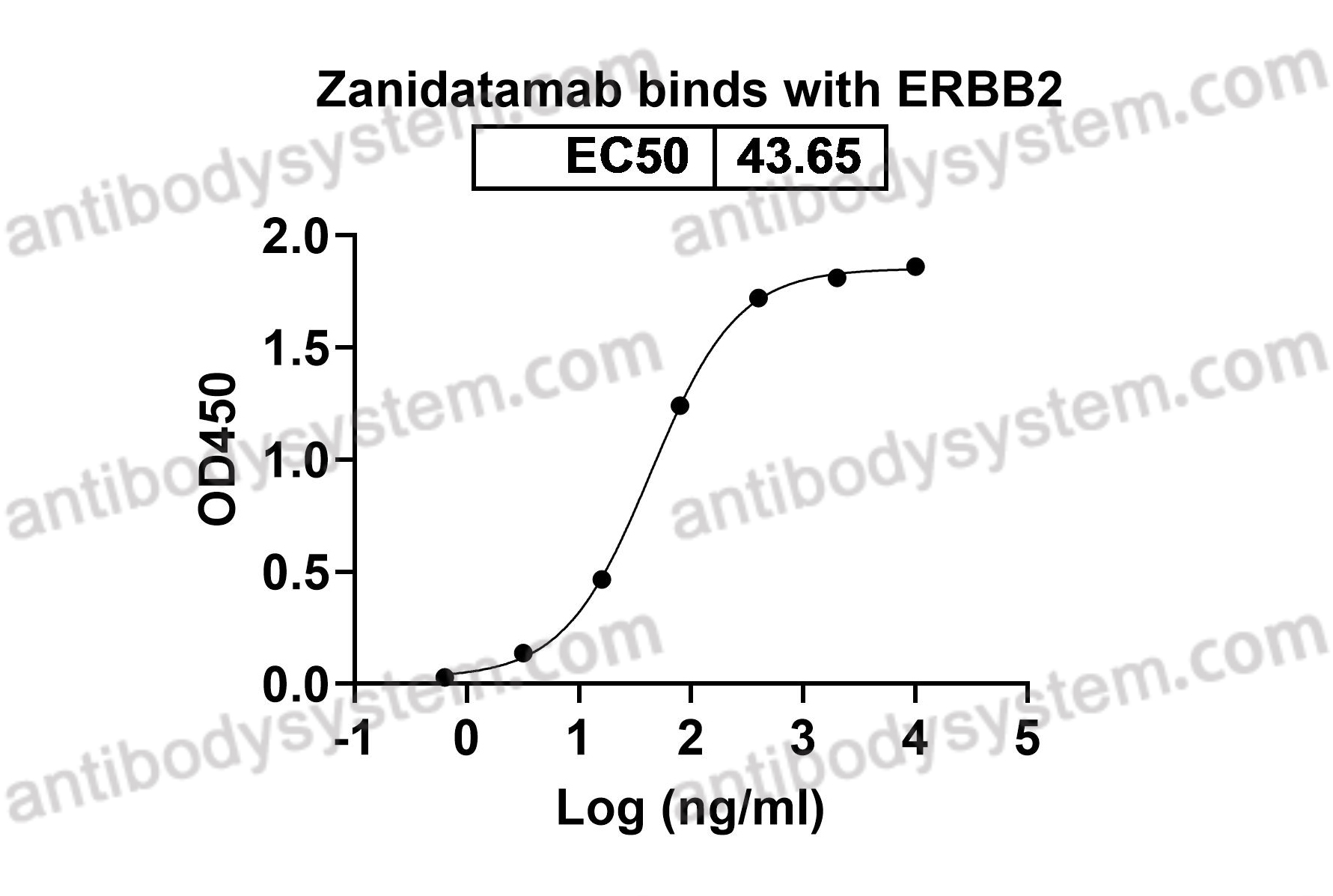
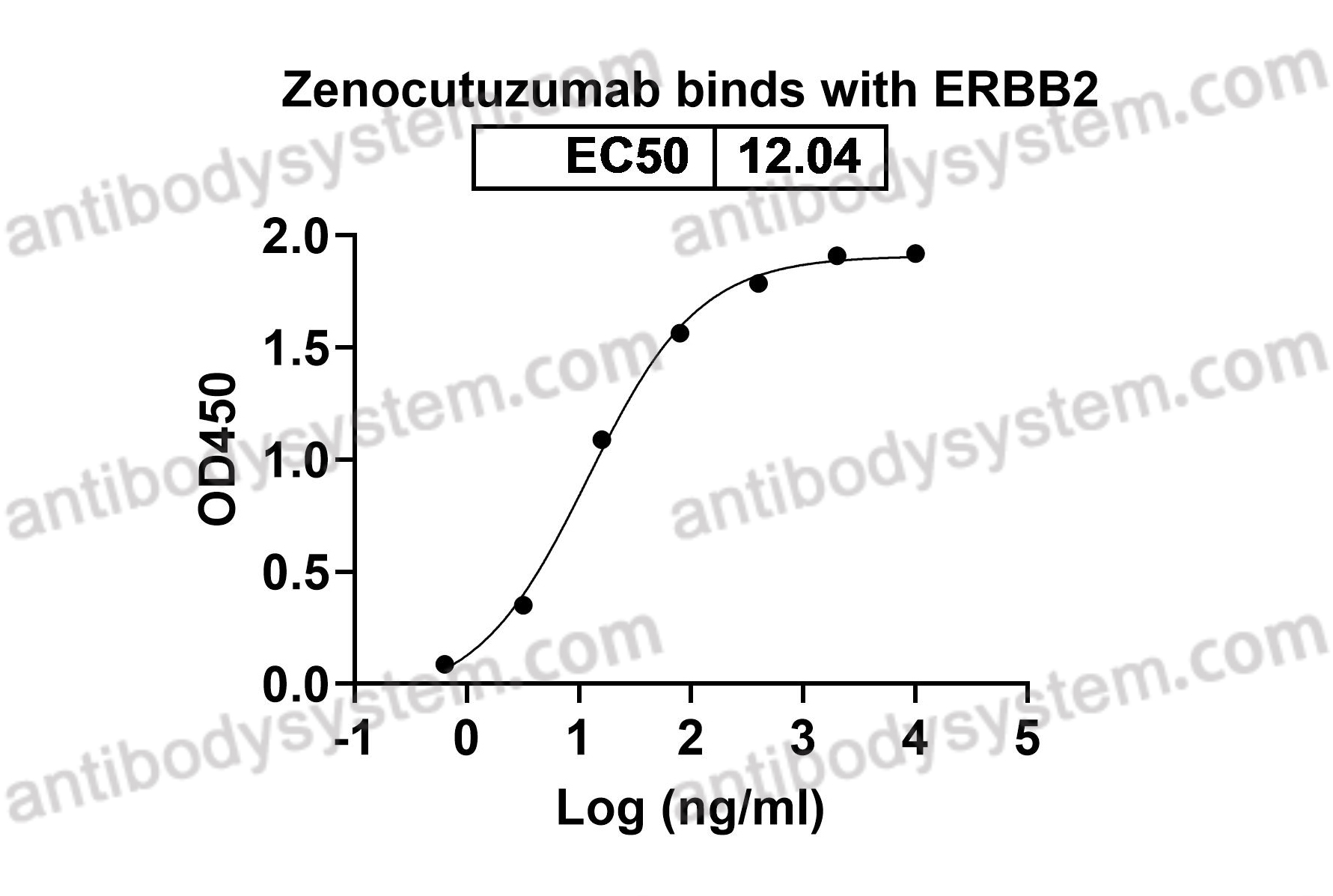
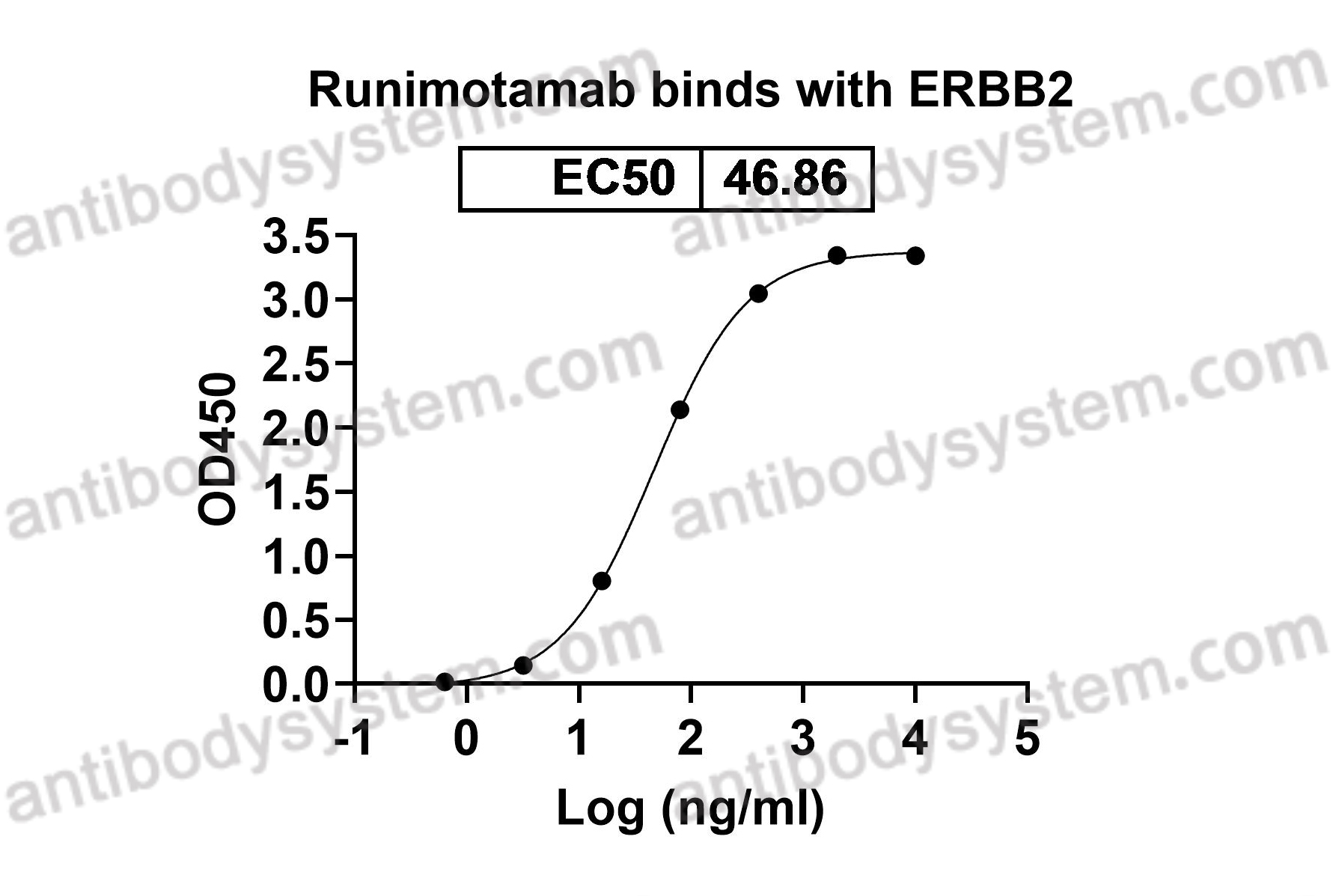
Catalog No.
EHC09601
Expression system
Mammalian Cells
Species
Homo sapiens (Human)
Protein length
Met1-Thr652
Predicted molecular weight
72.91 kDa
Nature
Recombinant
Endotoxin level
Please contact with the lab for this information.
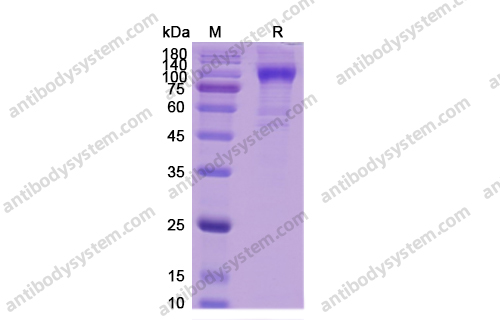
Purity
>90% as determined by SDS-PAGE.
Accession
P04626
Applications
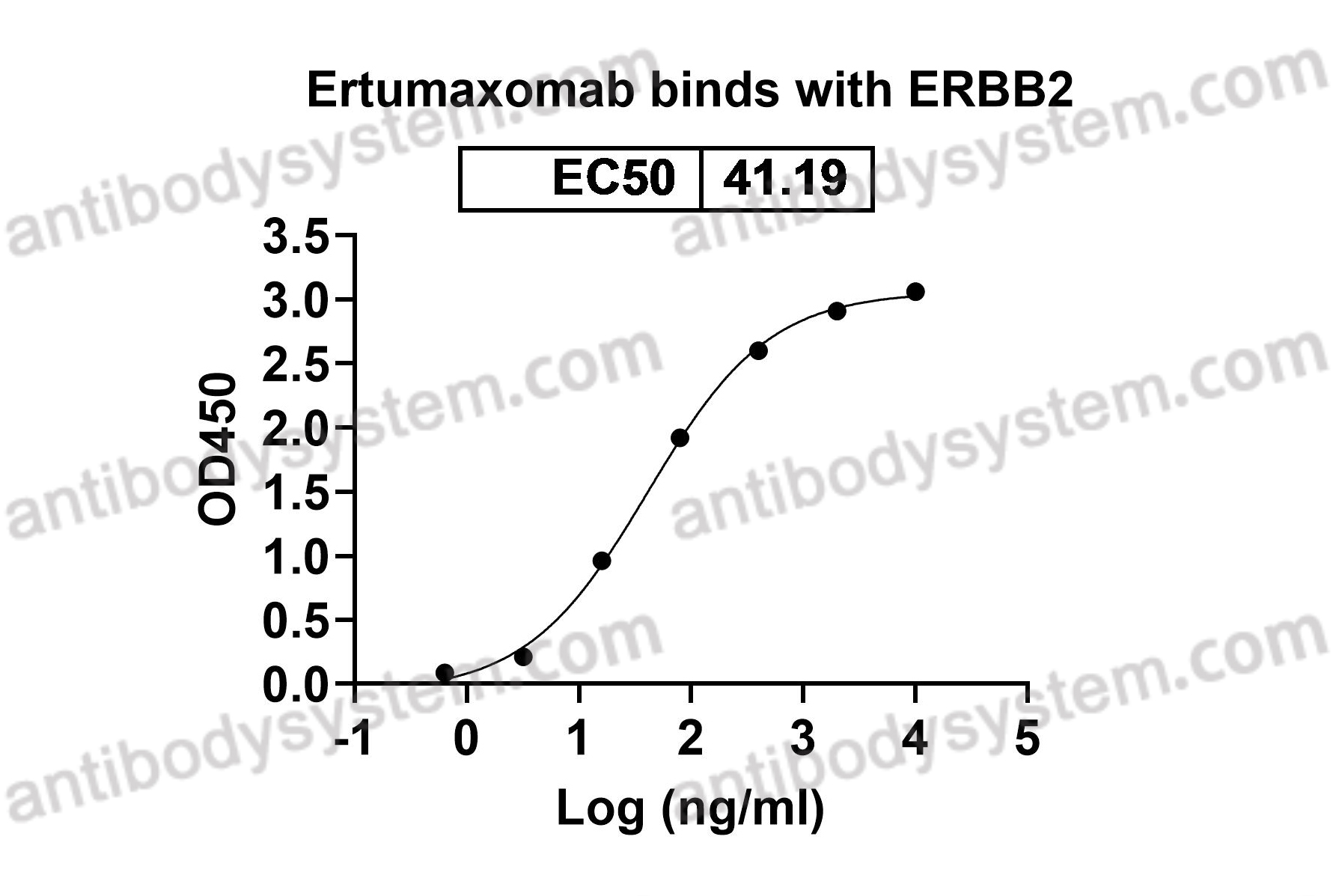
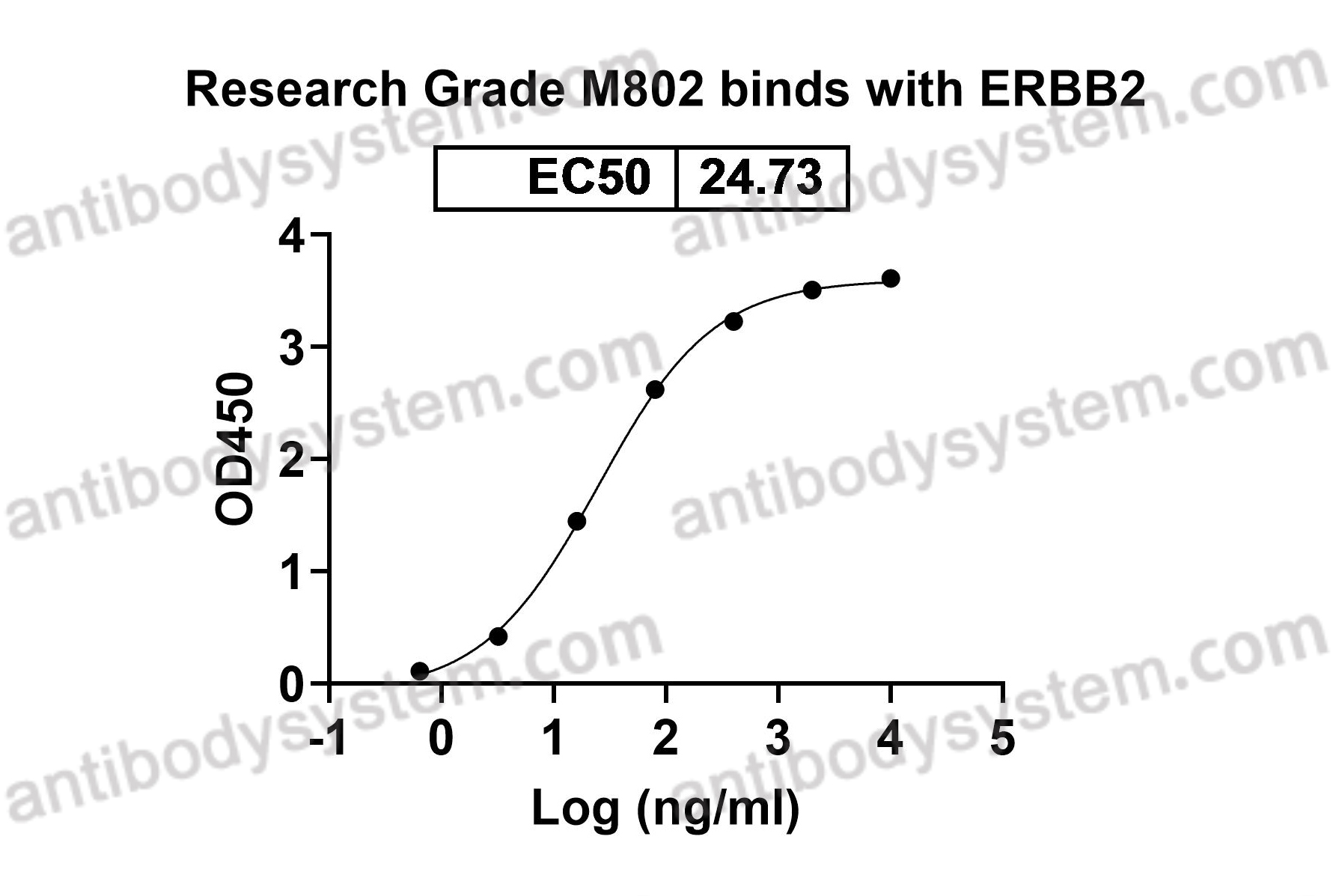
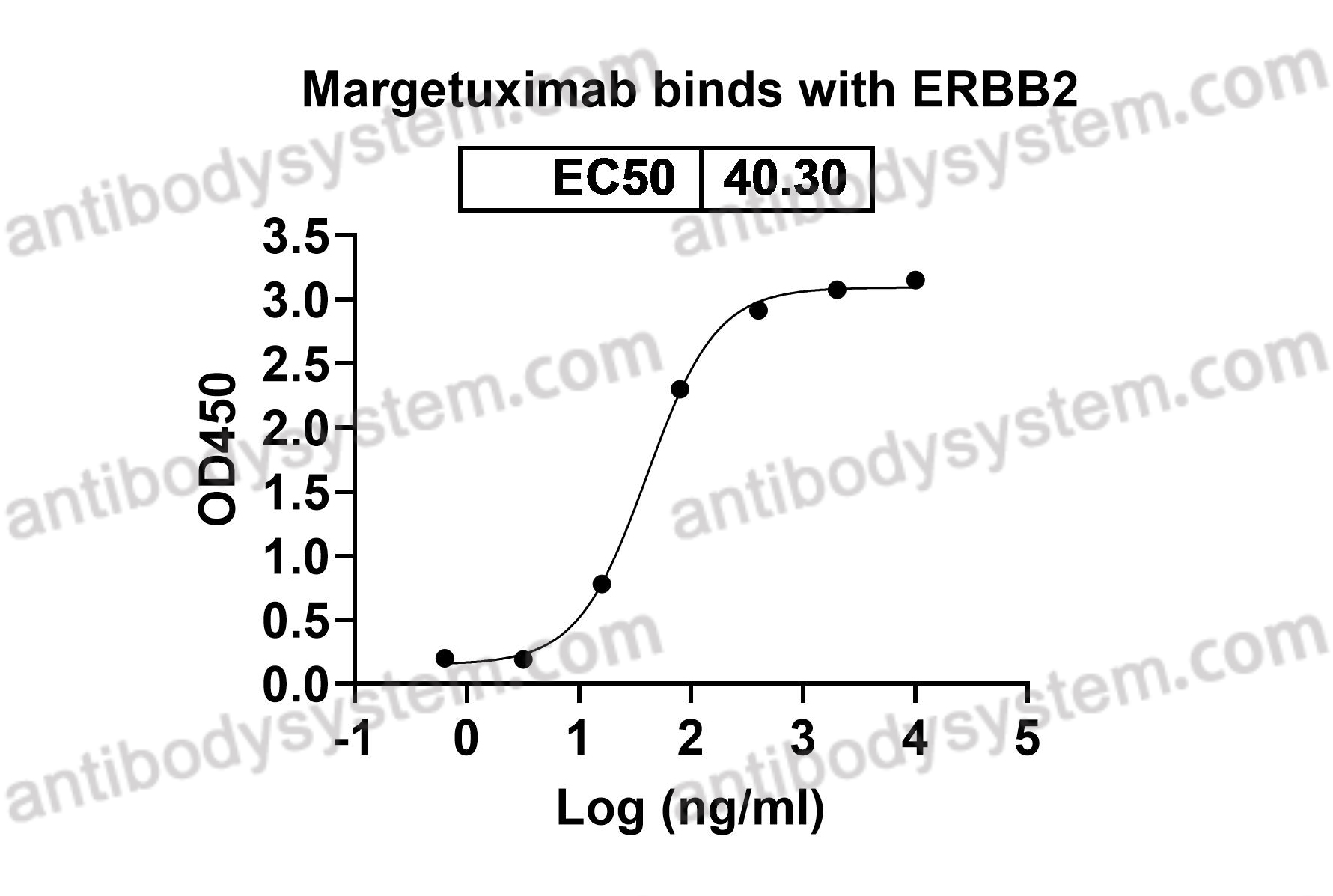
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Form
Lyophilized
Storage buffer
Lyophilized from a solution in PBS, pH 7.4, 5 % trehalose, 5 %mannitol.
Reconstitution
Reconstitute in sterile water for a stock solution.
Shipping
In general, proteins are provided as lyophilized powder/frozen liquid. They are shipped out with dry ice/blue ice unless customers require otherwise.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Alternative Names
p185erbB2, HER2, NGL, Tyrosine kinase-type cell surface receptor HER2, NEU, MLN 19, Proto-oncogene Neu, MLN19, ERBB2, Proto-oncogene c-ErbB-2, CD340, Receptor tyrosine-protein kinase erbB-2, Metastatic lymph node gene 19 protein
Immune Subtyping Identifies Patients With Hormone Receptor-Positive Early-Stage Breast Cancer Who Respond to Neoadjuvant Immunotherapy (IO): Results From Five IO Arms of the I-SPY2 Trial., PMID:40526879
Molecular landscape of HER2-mutated non-small cell lung cancer in Northeastern Brazil: Clinical, histopathological, and genomic insights., PMID:40526089
Real-world treatment patterns and outcomes of patients with hormone receptor-positive/HER2-low metastatic breast cancer treated with chemotherapy., PMID:40525913
Two-dimensional analysis of plasma-derived extracellular vesicles to determine the HER2 status in breast cancer patients., PMID:40524202
Clinical Efficacy and Safety of Apatinib Combined with Irinotecan in HER2-negative Patients with Advanced Gastric or Gastroesophageal Junction Adenocarcinoma after First-Line Treatment Failure: A Single-Arm, Single-Center Retrospective Study., PMID:40524072
Decoding the molecular landscape: HER2 and PD-L1 in advanced gastric cancer., PMID:40519936
Association of HER2-low with clinicopathological features in patients with early invasive lobular breast cancer: an international multicentric study., PMID:40514709
Prognostic impact of HER2-low expression in triple-negative breast cancer of high-grade special histological type and no special type., PMID:40512776
Hereditary breast cancer beyond BRCA: clinicopathological characteristics and long-term outcomes., PMID:40504267
RNF213-Dependent EGFR and HER2 Activation Regulates Specific Downstream Signaling Pathways in Human Cancer Cells., PMID:40503863
Impact of age and comorbidities on real-world outcomes in advanced breast cancer patients treated with palbociclib in first line: a nation-wide Danish retrospective study., PMID:40500959
ERBB2-Low Expression by Race and Ethnicity Among Patients With Triple-Negative Breast Cancer., PMID:40498483
Predictive power of tertiary lymphoid structure for prognosis and neoadjuvant chemotherapy response in HER2-positive breast cancer., PMID:40489875
Improved Sampling Method to Enhance the Positive Rate of HER2 Detection in Gastric Cancer., PMID:40485523
Axillary staging with 18F-FDG PET/CT in early breast cancer: impact of tumor subtypes., PMID:40482189
A homogeneous mass-encoded strategy for mass spectrometric biosensing of multiplex proteins., PMID:40480694
Advances and Future Perspectives of HER2 Mutations in Non-Small Lung Cancer (NSCLC), Especially in China., PMID:40472040
Research progress on HER2-specific chimeric antigen receptor T cells for immunotherapy of solid tumors., PMID:40469307
TCHL - a phase II neo-adjuvant study assessing TCH (docetaxel, carboplatin and trastuzumab) and TCHL (docetaxel, carboplatin, trastuzumab and lapatinib) in HER-2 positive breast cancer patients: a 5-year follow-up with serum biomarker analysis., PMID:40468999
Successful Treatment of HER2 V659E Mutation-Positive Lung Adenocarcinoma With Trastuzumab Deruxtecan: A Case Report., PMID:40468744
Radiation therapy in clinically node positive HER2 positive breast cancer after primary systemic therapy and breast conserving surgery: pooled analysis of TRYPHAENA and NeoSphere trials., PMID:40468250
Effect of HER2-low expression on the efficacy of neoadjuvant chemotherapy in triple-negative breast cancer., PMID:40467708
Targeting SLC7A11-mediated cysteine metabolism for the treatment of trastuzumab-resistant HER2-positive breast cancer., PMID:40464376
Mechanisms of resistance to antibody-drug conjugates in cancers., PMID:40460510
Assessing the Relationship among Tumor Size, Lymph Node Status, and ER/PR/HER2 Expression in Patients with Breast Cancer., PMID:40460385
T-cell activation enhances anti-HER2-mediated antibody-dependent cellular cytotoxicity in gastric cancer., PMID:40455140
Research advancements of antibody drug conjugates in non-small cell lung cancer with HER2 alterations., PMID:40448190
Chemically Engineered Affinity Protein Drugs for Covalent Targeted Cancer Therapy., PMID:40445865
Solution structure and synaptic analyses reveal determinants of bispecific T cell engager potency., PMID:40445758
Patient-reported outcomes with trastuzumab deruxtecan in hormone receptor-positive, HER2-low or HER2-ultralow metastatic breast cancer: results from the randomized DESTINY-Breast06 trial., PMID:40441802
Updated efficacy and safety of CDK4/6 inhibitors plus endocrine therapy in elderly women with HR+/HER-2 metastatic or advanced breast cancer: patient-level network meta-analysis., PMID:40440494
Metastatic breast cancer through the Oncotype DX Breast Recurrence Score®: insights from a small cohort study., PMID:40437301
Real-world data on trastuzumab emtansine (TDM1) efficacy and safety: Results of a single-centre retrospective study of HER2-positive metastatic breast cancer patients., PMID:40437155
Pyrotinib promotes the antitumor effect of T-DM1 by increasing drug endocytosis in HER2-positive breast cancer., PMID:40437017
Cost-effectiveness analysis of first-line cadonilimab plus chemotherapy in HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma., PMID:40433373
CD44 Marks Dormant Tumor Cells After HER2 Inhibition in Breast Cancer Cells., PMID:40430044
Impact of HER2 Status Assessed by Immunohistochemistry on Treatment Response in Patients with Metastatic Breast Cancer Receiving Trastuzumab Emtansine., PMID:40428777
LIFR-Mediated ERBB2 Signaling Is Essential for Successful Embryo Implantation in Mice., PMID:40427591
Hedgehog inhibitors exert anti-proliferation effects and synergistically interact with trastuzumab in HER2-positive gastric cancer models., PMID:40426308
Safety and quality of life of CDK4/6 inhibitors therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: a multicenter cross-sectional survey in China., PMID:40426093
Subtype-specific dysregulation of biogenic amine-related genes and miRNAs in breast cancer: identification of DRD2, HRH2, and HRH4 as potential therapeutic targets in TNBC and HER2+ subtypes., PMID:40425926
Antibody-drug conjugate (disitamab vedotin) therapy targeting HER2-low or higher advanced extramammary Paget's disease., PMID:40421956
Validated Immunochemical Assay for Comprehensive Determination of the Human Epidermal Growth Factor Receptor 2 Released from and Bound to Cells., PMID:40418656
Breast cancer early detection and molecular subtype prediction by combination of Raman spectroscopy with deep learning., PMID:40412234
Prevalence and clinicopathological features of human epidermal growth factor receptor-2-low breast cancers: A single-center experience., PMID:40411392
miRNA panel from HER2+ and CD24+ plasma extracellular vesicle subpopulations as biomarkers of early-stage breast cancer., PMID:40405296
Patient preferences for treatments in hormone receptor-positive/HER2-negative metastatic breast cancer in Italy: a discrete choice experiment study., PMID:40405072
Efficacy and safety of trastuzumab deruxtecan in HER2-positive breast cancer patients with brain metastases after failure of pyrotinib-based therapy., PMID:40404737
Targeting HER2 with DNA Aptamers for Efficient Anticancer Drug Delivery: A Combined Experimental and Computational Study., PMID:40403699
Intra-tumoral spatial heterogeneity in breast cancer quantified using high-dimensional protein multiplexing and single cell phenotyping., PMID:40399910