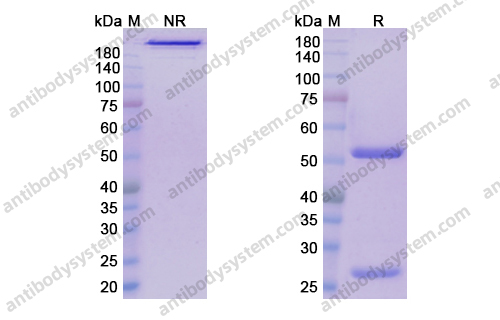
Overview
Catalog No.
DHD14201
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
PEMT, CA 15-3, H23AG, Peanut-reactive urinary mucin, PEM, EMA, Cancer antigen 15-3, MUC-1, Episialin, MUC1-CT, MUC1-beta, Tumor-associated mucin, CD227, Polymorphic epithelial mucin, KL-6, MUC1-alpha, MUC1, PUM, Krebs von den Lungen-6, MUC1-NT, Tumor-associated epithelial membrane antigen, Mucin-1, Breast carcinoma-associated antigen DF3, Carcinoma-associated mucin
Concentration
6.97 mg/ml
Endotoxin level
Please contact with the lab for this information.
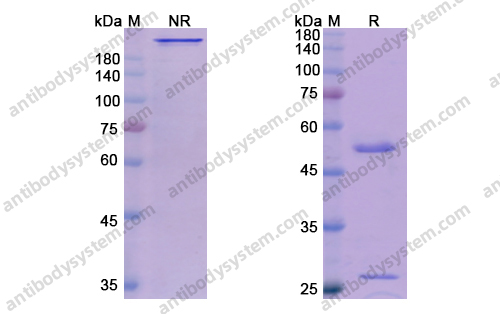
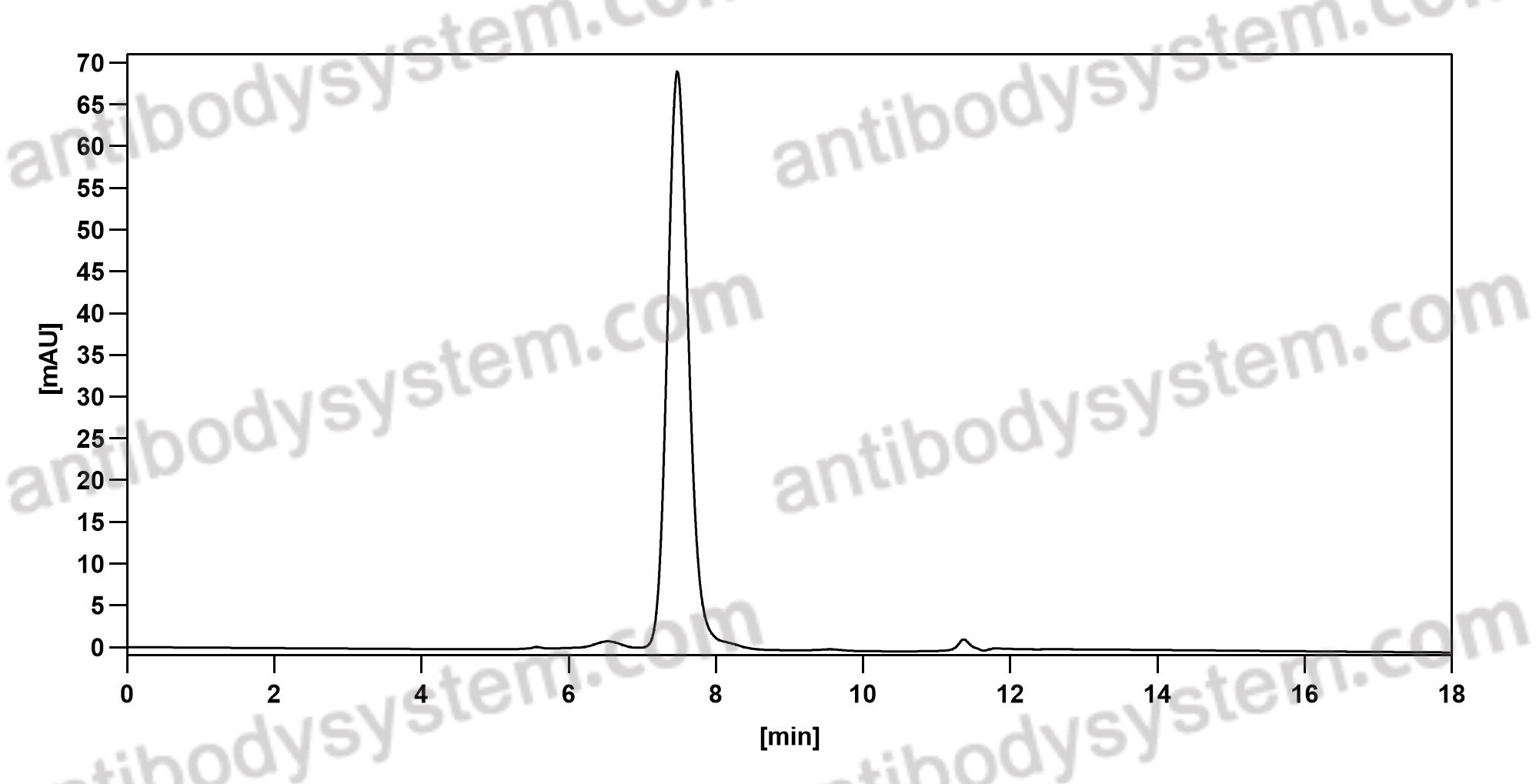
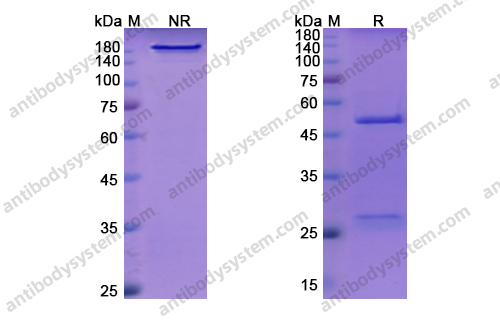
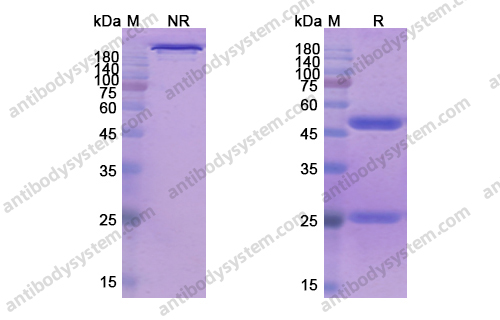
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P15941
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
100 mM Pro-Ac, 20 mM Arg, pH 5.0
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
PankoMab, PankoMab-GEX, hPankoMab, CAS: 1264737-26-9
Clone ID
Gatipotuzumab
Data Image
References
Potential Interplay of the Gatipotuzumab Epitope TA-MUC1 and Estrogen Receptors in Ovarian Cancer, PMID: 30642093
TA-MUC1 as detected by the fully humanized, therapeutic antibody Gatipotzumab predicts poor prognosis in cervical cancer, PMID: 30062487
EP3 receptor is a prognostic factor in TA-MUC1-negative ovarian cancer, PMID: 31485769
Erratum to 'Safety and preliminary activity results of the GATTO study, a phase Ib study combining the anti-TA-MUC1 antibody Gatipotuzumab with the anti-EGFR Tomuzotuximab in patients with refractory solid tumors': [ESMO Open Volume 7, Issue 2, April 2022, 100447]., PMID:35841804
Safety and preliminary activity results of the GATTO study, a phase Ib study combining the anti-TA-MUC1 antibody gatipotuzumab with the anti-EGFR tomuzotuximab in patients with refractory solid tumors., PMID:35397434
Maintenance therapy of patients with recurrent epithelial ovarian carcinoma with the anti-tumor-associated-mucin-1 antibody gatipotuzumab: results from a double-blind, placebo-controlled, randomized, phase II study., PMID:34920291
EP3 receptor is a prognostic factor in TA-MUC1-negative ovarian cancer., PMID:31485769
Potential Interplay of the Gatipotuzumab Epitope TA-MUC1 and Estrogen Receptors in Ovarian Cancer., PMID:30642093
TA-MUC1 as detected by the fully humanized, therapeutic antibody Gatipotzumab predicts poor prognosis in cervical cancer., PMID:30062487
Datasheet