Catalog No.
EVV03839
Expression system
Mammalian cells
Species
Influenza B virus (B/New Hampshire/01/2021)
Protein length
Ala15-Thr545
Predicted molecular weight
64.77 kDa
Nature
Recombinant
Endotoxin level
Please contact with the lab for this information.
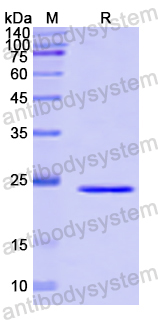
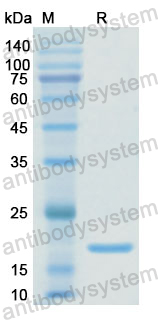
Purity
>90% as determined by SDS-PAGE.
Accession
QXN15658.1
Applications
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Form
Lyophilized
Storage buffer
Lyophilized from a solution in PBS pH 7.4, 1mM EDTA, 4% Trehalose, 1% Mannitol.
Reconstitution
Reconstitute in sterile water for a stock solution. A copy of datasheet will be provided with the products, please refer to it for details.
Shipping
In general, proteins are provided as lyophilized powder/frozen liquid. They are shipped out with dry ice/blue ice unless customers require otherwise.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for one week. Store at -20 to -80°C for twelve months from the date of receipt.
Alternative Names
Hemagglutinin, Hemagglutinin HA1 chain, Hemagglutinin HA2 chain, HA
Multiple antiviral mechanisms of Ephedrae Herba and Cinnamomi Cortex against influenza: inhibition of entry and replication., PMID:40304468
Influenza B viruses are more susceptible to high temperatures than influenza A viruses., PMID:40295766
Sulfatide Binds to Influenza B Virus and Enhances Viral Replication., PMID:40284974
Dual roles of influenza B virus neuraminidase mRNA vaccine in enhancing cross-lineage protection by supplementing inactivated split vaccination., PMID:40265888
Influenza A hemagglutinin virus-like particles confer protection against influenza B virus infection., PMID:40230200
A monoclonal anti-hemagglutinin stem antibody modified with zanamivir protects against both influenza A and B viruses., PMID:40193611
Tracking Seasonal Influenza Trends in South Tyrol During 2022/2023 Using Genomic Surveillance Data., PMID:40143444
Selection and identification of an ssDNA aptamer against influenza B virus hemagglutinin protein., PMID:40050943
A novel tetravalent influenza vaccine based on one chimpanzee adenoviral vector., PMID:40023902
Adenoviral-Vectored Multivalent Vaccine Provides Durable Protection Against Influenza B Viruses from Victoria-like and Yamagata-like Lineages., PMID:40004004
A single mutation at position 214 of influenza B hemagglutinin enhances cross-neutralization., PMID:39960410
Host-microbe multiomic profiling identifies distinct COVID-19 immune dysregulation in solid organ transplant recipients., PMID:39794319
Development of broadly protective influenza B vaccines., PMID:39774170
Antiviral Effect of Amentoflavone Against Influenza Viruses., PMID:39596490
Hemagglutination-Inhibition Antibodies and Protection against Influenza Elicited by Inactivated and Live Attenuated Vaccines in Children., PMID:39504434
Collaborative study on the cross-reactivity of two influenza B viral components in single radial immunodiffusion assay using quadrivalent influenza vaccines in Japan from 2015/16 to 2021-22 influenza season., PMID:39481190
Engineering a cleaved, prefusion-stabilized influenza B virus hemagglutinin by identification and locking of all six pH switches., PMID:39445049
Improved influenza vaccine responses after expression of multiple viral glycoproteins from a single mRNA., PMID:39379405
Molecular epidemiology and vaccine compatibility analysis of seasonal influenza A viruses in the context of COVID-19 epidemic in Wuhan, China., PMID:39370830
Triton X-100-treated virus-based ELLA demonstrates discordant antigenic evolution of influenza B virus hemagglutinin and neuraminidase., PMID:39360825
Influenza B Virus Receptor Specificity: Closing the Gap between Binding and Tropism., PMID:39339833
Antigenic analysis of the influenza B virus hemagglutinin protein., PMID:39233140
The evolutionary features and roles of single nucleotide variants and charged amino acid mutations in influenza outbreaks during NPI period., PMID:39223292
Evaluation of a qualified MDCK cell line for virus isolation to develop cell-based influenza vaccine viruses with appropriate antigenicity., PMID:39213922
Intranasal administration of octavalent next-generation influenza vaccine elicits protective immune responses against seasonal and pre-pandemic viruses., PMID:39171925
Disulfide-stabilized trimeric hemagglutinin ectodomains provide enhanced heterologous influenza protection., PMID:39101691
Safety and Immunogenicity of Respiratory Syncytial Virus Prefusion F Protein Vaccine when Co-administered with Adjuvanted Seasonal Quadrivalent Influenza Vaccine in Older Adults: A Phase 3 Randomized Trial., PMID:39099085
Molecular epidemiology and phylogenetic analysis of influenza viruses A (H3N2) and B/Victoria during the COVID-19 pandemic in Guangdong, China., PMID:39090685
Genomic evolution of influenza during the 2023-2024 season, the johns hopkins health system., PMID:39079210
Resurgence of influenza with increased genetic diversity of circulating viruses during the 2022-2023 season., PMID:39073070
Genetic Reassortment in a Child Coinfected with Two Influenza B Viruses, B/Yamagata Lineage and B/Victoria-Lineage Strains., PMID:38932274
HA antigenic variation and phylogenetic analysis of influenza B virus in Shiraz, Iran., PMID:38897314
Influenza Virus Genomic Surveillance, Arizona, USA, 2023-2024., PMID:38793574
Host-microbe multiomic profiling reveals age-dependent immune dysregulation associated with COVID-19 immunopathology., PMID:38630846
An Investigation of Severe Influenza Cases in Russia during the 2022-2023 Epidemic Season and an Analysis of HA-D222G/N Polymorphism in Newly Emerged and Dominant Clade 6B.1A.5a.2a A(H1N1)pdm09 Viruses., PMID:38276147
Clinical characteristics of outpatients with influenza-B-associated pneumonia and molecular evolution of influenza B virus in Beijing, China, during the 2021-2022 influenza season., PMID:38233704
Protective human antibodies against a conserved epitope in pre- and postfusion influenza hemagglutinin., PMID:38147556
Influenza B virus neuraminidase: a potential target for next-generation vaccines?, PMID:38037386
Establishment of Reference Reagents for Single-Radial-Immunodiffusion Assay on the 2022/23 Seasonal Influenza Vaccine in Japan and Their Quality Validation., PMID:38030271
Existing Evidence for Influenza B Virus Adaptations to Drive Replication in Humans as the Primary Host., PMID:37896807
Palmitoylation of the hemagglutinin of influenza B virus by ER-localized DHHC enzymes 1, 2, 4, and 6 is required for efficient virus replication., PMID:37792001
A computationally optimized broadly reactive hemagglutinin vaccine elicits neutralizing antibodies against influenza B viruses from both lineages., PMID:37741893
Fc-Effector-Independent in vivo Activity of a Potent Influenza B Neuraminidase Broadly Neutralizing Antibody., PMID:37515226
ADCC: An underappreciated correlate of cross-protection against influenza?, PMID:36911705
Anti-neuraminidase and anti-hemagglutinin immune serum can confer inter-lineage cross protection against recent influenza B., PMID:36689429
Infant Antibody Repertoires during the First Two Years of Influenza Vaccination., PMID:36314798
Impact of adjuvant: Trivalent vaccine with quadrivalent-like protection against heterologous Yamagata-lineage influenza B virus., PMID:36248851
Substitutions near the HA receptor binding site explain the origin and major antigenic change of the B/Victoria and B/Yamagata lineages., PMID:36215486
Molecular characterization of haemagglutinin genes of influenza B viruses circulating in Ghana during 2016 and 2017., PMID:36149889
Molecular characterization and evolution dynamics of influenza B viruses circulating in Germany from season 1996/1997 to 2019/2020., PMID:36096395