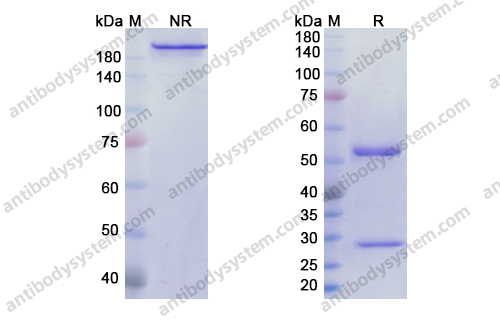
Catalog No.
DHD71901
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
KLRC1, NKG2-A/NKG2-B type II integral membrane protein, NKG2-A/B-activating NK receptor, CD159 antigen-like family member A, CD159a, NK cell receptor A, NKG2A
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
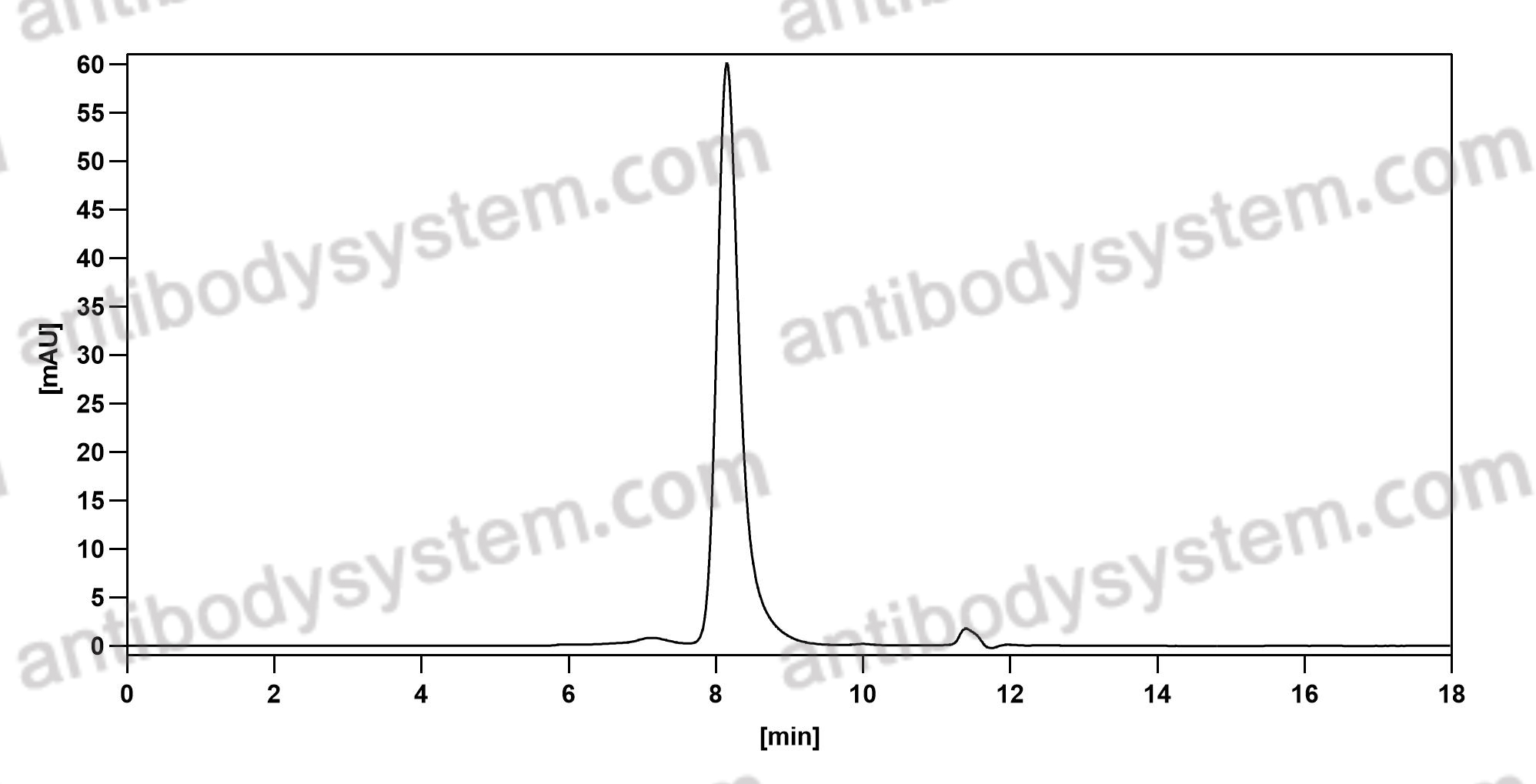
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P26715
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
IPH-2201, NN-8765, NNC-0141-0000-0100, NNC-141-01000, monolizumab,anti-NKG2A, humZ270, CAS: 1228763-95-8
Clone ID
Monalizumab
Monalizumab: inhibiting the novel immune checkpoint NKG2A, PMID: 31623687
Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells, PMID: 30503213
The NKG2A-HLA-E Axis as a Novel Checkpoint in the Tumor Microenvironment, PMID: 32409305
Immunotherapy Breakthroughs in the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma, PMID: 32967162
Dose-Ranging and Cohort-Expansion Study of Monalizumab (IPH2201) in Patients with Advanced Gynecologic Malignancies: A Trial of the Canadian Cancer Trials Group (CCTG): IND221, PMID: 31308062
Innate immunity in COVID-19 patients mediated by NKG2A receptors, and potential treatment using Monalizumab, Cholroquine, and antiviral agents, PMID: 32344314
Quantitative modeling predicts competitive advantages of a next generation anti-NKG2A monoclonal antibody over monalizumab for the treatment of cancer, PMID: 33501768
[New frontiers in the fight against cancer], PMID: 30973133
Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia, PMID: 27853650
Born to Kill: NK Cells Go to War against Cancer, PMID: 30898260
High-Density of FcγRIIIA + (CD16 +) Tumor-Associated Neutrophils in Metastases Improves the Therapeutic Response of Cetuximab in Metastatic Colorectal Cancer Patients, Independently of the HLA-E/CD94-NKG2A Axis, PMID: 34211852
Targeting NKG2A to elucidate natural killer cell ontogenesis and to develop novel immune-therapeutic strategies in cancer therapy, PMID: 30645023
Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trial., PMID:40450142
A phase II study of monalizumab and durvalumab in patients with recurrent/metastatic squamous cell carcinoma of the head and neck: results of the I2 cohort of the EORTC-HNCG-1559 trial (UPSTREAM)., PMID:40311183
INTERLINK-1: A Phase III, Randomized, Placebo-Controlled Study of Monalizumab Plus Cetuximab in Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma., PMID:40300079
Nationwide multi-centric prospective study for the identification of biomarkers to predict the treatment responses of nivolumab through comprehensive analyses of pretreatment plasma exosome mRNAs from head and neck cancer patients (BIONEXT study)., PMID:39867897
Generation, Characterization, and Preclinical Studies of a Novel NKG2A-Targeted Antibody BRY805 for Cancer Immunotherapy., PMID:39584993
PACIFIC-9: Phase III trial of durvalumab + oleclumab or monalizumab in unresectable stage III non-small-cell lung cancer., PMID:39023287
IFNγ mediates the resistance of tumor cells to distinct NK cell subsets., PMID:38955423
Combined IL6 and CCR2 blockade potentiates antitumor activity of NK cells in HPV-negative head and neck cancer., PMID:38468260
Phase 1/2 study of monalizumab plus durvalumab in patients with advanced solid tumors., PMID:38309722
Intratumoral co-injection of NK cells and NKG2A-neutralizing monoclonal antibodies., PMID:37782273
Neoadjuvant Durvalumab Alone or Combined with Novel Immuno-Oncology Agents in Resectable Lung Cancer: The Phase II NeoCOAST Platform Trial., PMID:37707791
A Novel Natural Killer Cell-related Gene Signature for Improving the Prediction of Prognosis and Immunotherapy Response in Bladder Cancer., PMID:37653625
Inhibitory NKG2A+ and absent activating NKG2C+ NK cell responses are associated with the development of EBV+ lymphomas., PMID:37426645
Unleashing NK- and CD8 T cells by combining monalizumab and trastuzumab for metastatic HER2-positive breast cancer: Results of the MIMOSA trial., PMID:37393645
Immunotherapy in Head and Neck Cancer: Where Do We Stand?, PMID:37213060
Population Pharmacokinetics of Monalizumab in Patients With Advanced Solid Tumors., PMID:36852723
NKG2A Immune Checkpoint in Vδ2 T Cells: Emerging Application in Cancer Immunotherapy., PMID:36831606
Killer to cure: Expression and production costs calculation of tobacco plant-made cancer-immune checkpoint inhibitors., PMID:36811226
NKG2A blocks the anti-metastatic functions of natural killer cells., PMID:36787695
Monalizumab efficacy correlates with HLA-E surface expression and NK cell activity in head and neck squamous carcinoma cell lines., PMID:36547689
NKG2A-checkpoint inhibition and its blockade critically depends on peptides presented by its ligand HLA-E., PMID:35596615
COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non-Small-Cell Lung Cancer., PMID:35452273
Phase I Study of Safety, Tolerability, and Efficacy of Tebentafusp Using a Step-Up Dosing Regimen and Expansion in Patients With Metastatic Uveal Melanoma., PMID:35254876
NK cell-based therapies for HIV infection: Investigating current advances and future possibilities., PMID:34668588
A phase II study of monalizumab in patients with recurrent/metastatic squamous cell carcinoma of the head and neck: The I1 cohort of the EORTC-HNCG-1559 UPSTREAM trial., PMID:34638090
High-Density of FcγRIIIA+ (CD16+) Tumor-Associated Neutrophils in Metastases Improves the Therapeutic Response of Cetuximab in Metastatic Colorectal Cancer Patients, Independently of the HLA-E/CD94-NKG2A Axis., PMID:34211852
Quantitative modeling predicts competitive advantages of a next generation anti-NKG2A monoclonal antibody over monalizumab for the treatment of cancer., PMID:33501768
Immunotherapy Breakthroughs in the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma., PMID:32967162
The NKG2A-HLA-E Axis as a Novel Checkpoint in the Tumor Microenvironment., PMID:32409305
Innate immunity in COVID-19 patients mediated by NKG2A receptors, and potential treatment using Monalizumab, Cholroquine, and antiviral agents., PMID:32344314
Monalizumab: inhibiting the novel immune checkpoint NKG2A., PMID:31623687
Dose-Ranging and Cohort-Expansion Study of Monalizumab (IPH2201) in Patients with Advanced Gynecologic Malignancies: A Trial of the Canadian Cancer Trials Group (CCTG): IND221., PMID:31308062
Born to Kill: NK Cells Go to War against Cancer., PMID:30898260
Targeting NKG2A to elucidate natural killer cell ontogenesis and to develop novel immune-therapeutic strategies in cancer therapy., PMID:30645023
Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells., PMID:30503213
[New frontiers in the fight against cancer]., PMID:30973133
Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia., PMID:27853650