[Psoriatic arthritis : Drugs of the (near) future], PMID: 28681115
A revolutionary therapeutic approach for psoriasis: bispecific biological agents, PMID: 27153320
An Overview of Bimekizumab for the Treatment of Psoriatic Arthritis: The Evidence so Far, PMID: 33727793
Anti-IL-17 Agents in the Treatment of Axial Spondyloarthritis, PMID: 33977094
Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial, PMID: 33549192
Bimekizumab for Moderate-to-Severe Plaque Psoriasis, PMID: 34077151
Bimekizumab for patients with moderate to severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled, phase 2b extension study, PMID: 32473974
Bimekizumab for the treatment of psoriatic disease, PMID: 30332893
Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial, PMID: 32035552
Bimekizumab Offers Rapid and Durable Psoriasis Treatment, PMID: 33904869
Bimekizumab versus Adalimumab in Plaque Psoriasis, PMID: 33891379
Bimekizumab versus Secukinumab in Plaque Psoriasis, PMID: 33891380
Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial, PMID: 33549193
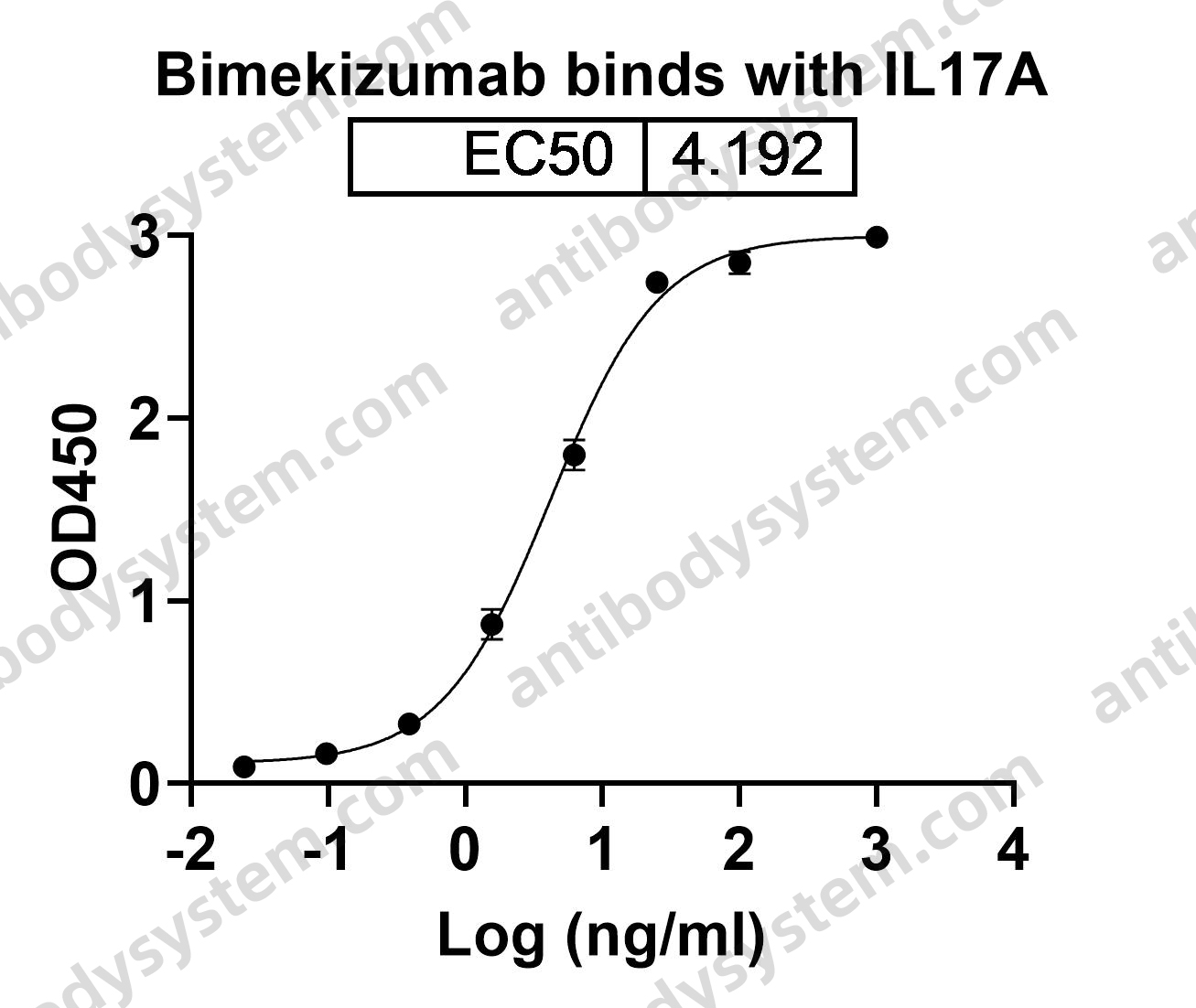
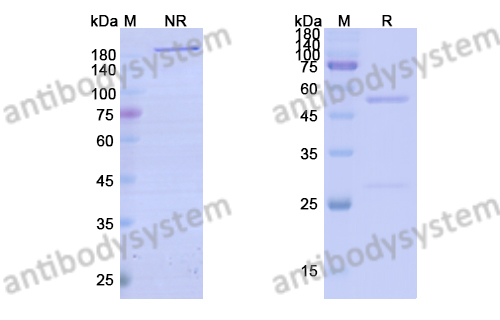
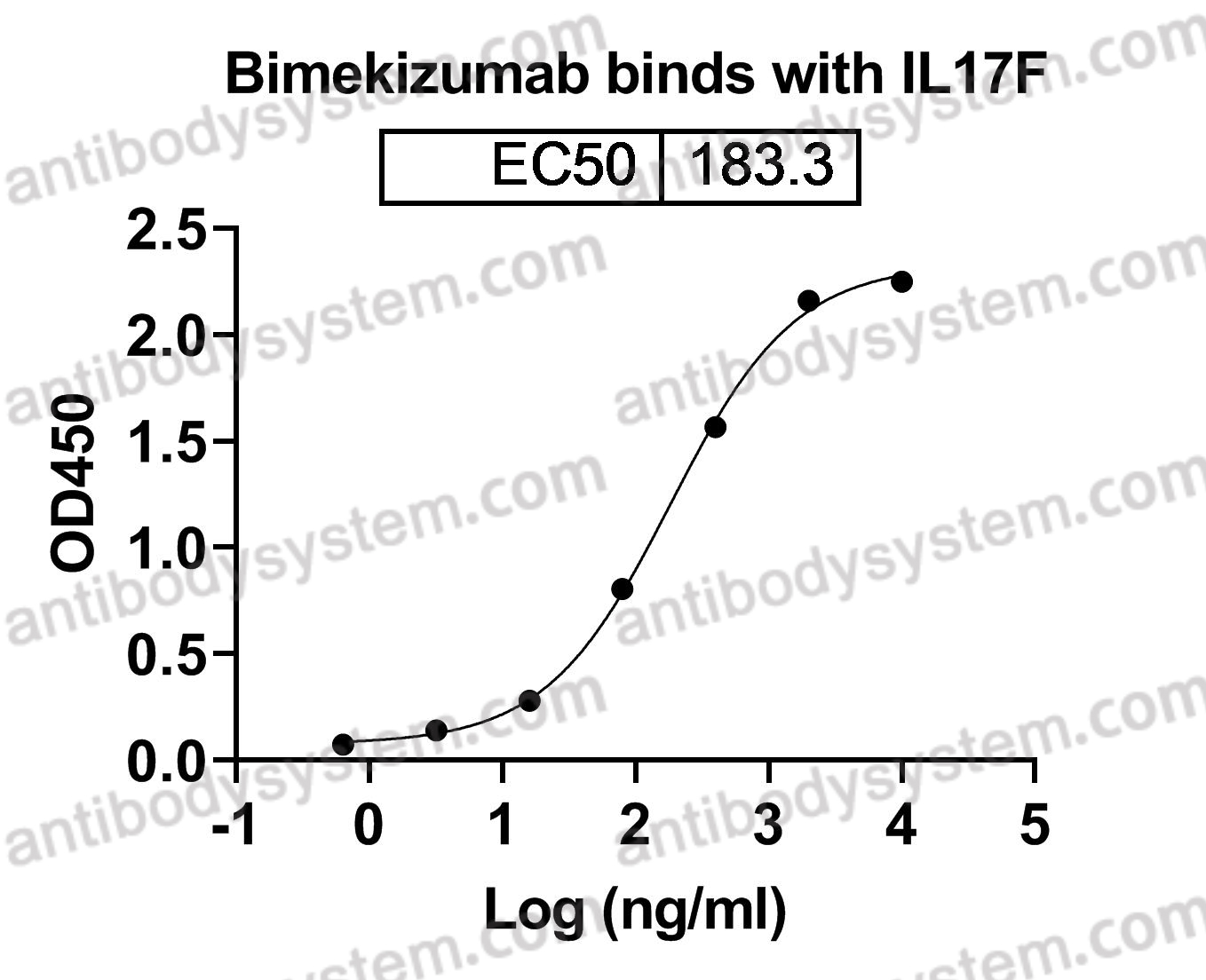
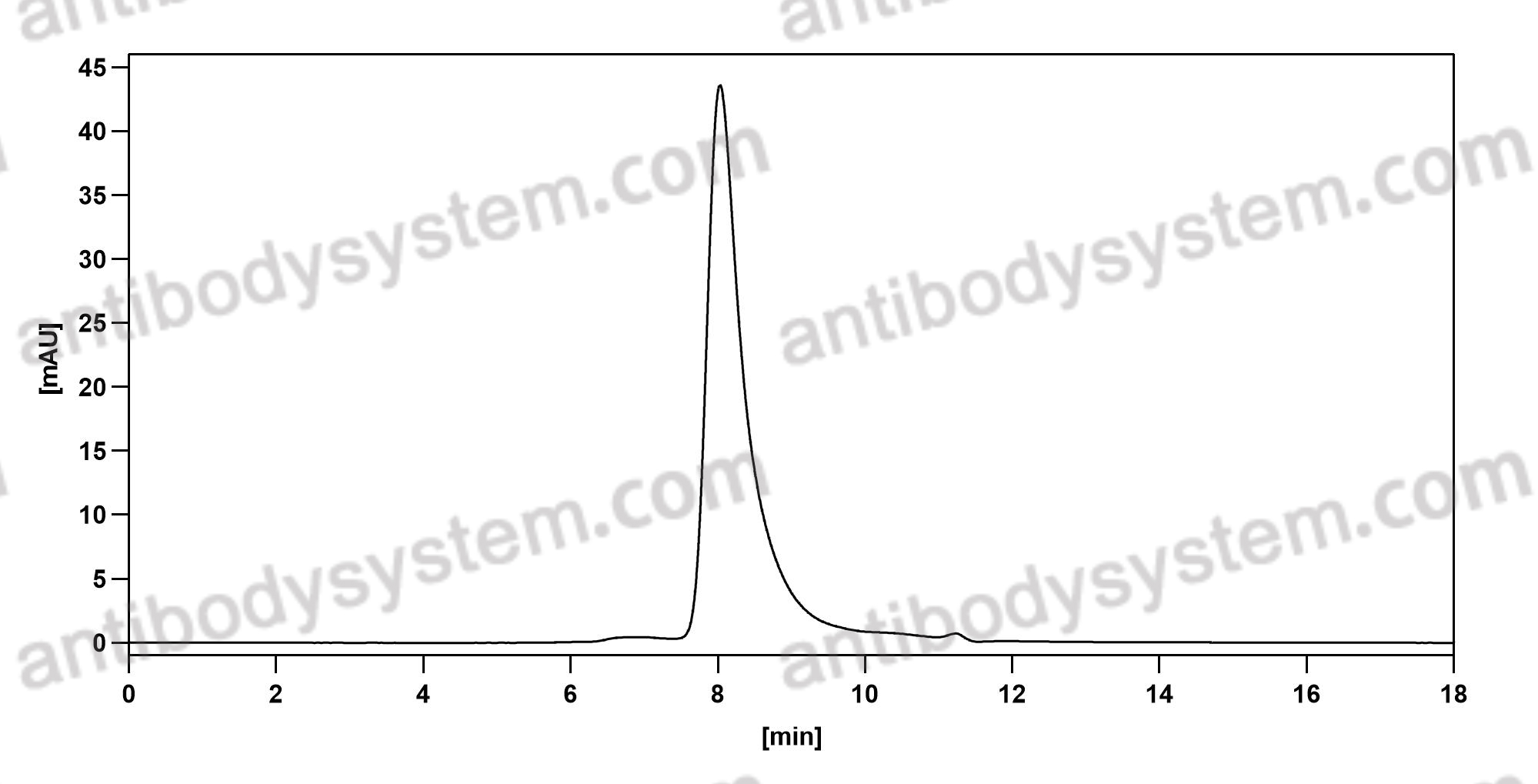
Bimekizumab, a Novel Humanized IgG1 Antibody That Neutralizes Both IL-17A and IL-17F, PMID: 32973785
Bimekizumab: a dual IL-17A and IL-17F inhibitor for the treatment of psoriasis and psoriatic arthritis, PMID: 34384327
Bimekizumab: The First Dual Inhibitor of Interleukin (IL)-17A and IL-17F for the Treatment of Psoriatic Disease and Ankylosing Spondylitis, PMID: 31172372
Bimekizumab: the new drug in the biologics armamentarium for psoriasis, PMID: 34178093
Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management, PMID: 33026212
Comparative efficacy and safety of biologics in moderate to severe plaque psoriasis: a multiple-treatments meta-analysis, PMID: 33377312
Correction: Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase llb, randomised, double blind, placebo-controlled, dose-ranging study, PMID: 32816784
Development and Content Validation of the Psoriasis Symptoms and Impacts Measure (P-SIM) for Assessment of Plaque Psoriasis, PMID: 32844372
Developments with experimental and investigational drugs for axial spondyloarthritis, PMID: 28562100
Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation, PMID: 29275332
Dual inhibition of IL-17A and IL-17F in psoriatic disease, PMID: 34408825
Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells, PMID: 32723833
Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study, PMID: 32253184
Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: Results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial, PMID: 29609013
Efficacy and safety of bimekizumab as add-on therapy for rheumatoid arthritis in patients with inadequate response to certolizumab pegol: a proof-of-concept study, PMID: 31177099
Efficacy and Safety of Bimekizumab in Moderate to Severe Hidradenitis Suppurativa: A Phase 2, Double-blind, Placebo-Controlled Randomized Clinical Trial, PMID: 34406364
Efficacy and safety of interleukin-17A inhibitors in patients with ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials, PMID: 33432451
Emerging drugs for the treatment of axial spondyloarthritis, PMID: 29475394
Emerging systemic drugs in the treatment of plaque psoriasis, PMID: 32192366
Emerging treatment options for spondyloarthritis, PMID: 31171316
Emerging treatment options for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis: evaluating bimekizumab and its therapeutic potential [Corrigendum], PMID: 31616625
Emerging treatment options for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis: evaluating bimekizumab and its therapeutic potential, PMID: 31214486
European League Against Rheumatism And American Diabetes Association, PMID: 27504068
First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis, PMID: 27859546
IL-17 inhibition in axial spondyloarthritis: current and future perspectives, PMID: 30957574
Inhibition of interleukins 17A and 17F in psoriatic arthritis, PMID: 32035535
Latest Advances for the Treatment of Chronic Plaque Psoriasis with Biologics and Oral Small Molecules, PMID: 34239295
Mini Review: New Treatments in Psoriatic Arthritis. Focus on the IL-23/17 Axis, PMID: 31447673
Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis?, PMID: 28765121
Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review, PMID: 32427307
Pharmacological development in hidradenitis suppurativa, PMID: 31075754
State of the art and pharmacological pipeline of biologics for chronic plaque psoriasis, PMID: 31212119
Systematic review of immunomodulatory therapies for hidradenitis suppurativa, PMID: 31190730
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis, PMID: 31917873
The next quantum leap forward? Bimekizumab for psoriasis, PMID: 33549176
Treating to Target(s) With Interleukin-17 Inhibitors, PMID: 30742778
Treatment of psoriasis: janus kinases inhibitors and biologics for the interleukin-23/Th17 axis, PMID: 32166932
An evaluation of bimekizumab for the treatment of hidradenitis suppurativa., PMID:40524676
A network meta-analysis study of monotherapies for hidradenitis suppurativa: analyses of the current evidence base., PMID:40464762
[Not Available]., PMID:40417903
Biologic and Non-Biologic Therapies for Scalp Psoriasis: A Network Meta-analysis of Randomized Controlled Trials., PMID:40401874
Biologic therapies and small molecules in the treatment of hidradenitis suppurativa., PMID:40373949
Correction to: The risk of candidiasis during treatment with bimekizumab for the management of plaque psoriasis: a 16-week multicentre real-world experience - IL PSO (Italian Landscape Psoriasis)., PMID:40373079
Super Responder Profile Under Bimekizumab Treatment in Moderate-to-Severe Psoriasis: A Short Term Real-Life Observation-IL PSO (Italian Landscape Psoriasis)., PMID:40372625
Gender Influence on Bimekizumab Response in Patients with Psoriasis: Results of a Real-World Multicenter Retrospective Study-IL PSO (Italian Landscape PSOriasis)., PMID:40358828
Summary of Research: Safety and Efficacy of Bimekizumab in Patients with Psoriatic Arthritis: 2-Year Results from Two Phase 3 Studies., PMID:40347389
Correction: Safety and Efficacy of Bimekizumab in Patients with Psoriatic Arthritis: 2-Year Results from Two Phase 3 Studies., PMID:40323371
Editor's Highlights-June 2025., PMID:40322884
Emerging manifestations of IL-17 immunomodulation in the gastrointestinal tract., PMID:40319948
Antifungal Prophylaxis and Effective Therapy With Bimekizumab in a Patient With Multiresistant Plaque Psoriasis and Recurrent Candidiasis., PMID:40304262
Bimekizumab durability of efficacy through 196 weeks and safety through 4 years in patients with moderate to severe plaque psoriasis: Results from the BE BRIGHT open-label extension trial., PMID:40286813
Adverse events associated with bimekizumab for moderate-to-severe plaque psoriasis: A retrospective, multicenter, post-hoc analysis., PMID:40276786
The role of interleukin inhibitors in the treatment of hidradenitis suppurativa; a systematic review of clinical trials., PMID:40268126
Acne Conglobata and Bimekizumab., PMID:40266588
Active Switch From a Single Dose of Bimekizumab to Risankizumab in Psoriasis: A Pilot Study., PMID:40260930
Bimekizumab as-needed dosing in patients with psoriasis: a case series., PMID:40256854
Real-World Experience of Bimekizumab in a Cohort of 109 Patients Over 48 Weeks and Identification of Predictive Factors for an Early Super Response and Risk of Adverse Events., PMID:40242569
Evaluating the Efficacy of Bimekizumab across the Different Sensitive Areas in Moderate-to-Severe Psoriasis: A 52-week Italian Multicenter Real-Life Lazio Experience., PMID:40228172
Long-term safety of bimekizumab in adult patients with axial spondyloarthritis or psoriatic arthritis: pooled results from integrated phase IIb/III clinical studies., PMID:40194794
Wolf's Isotopic Response: A Rare Case of Terra Firma-Forme Dermatosis on Resolved Psoriatic Plaques., PMID:40189743
Psychometric Validation and Interpretation Thresholds of the Hidradenitis Suppurativa Quality of Life (HiSQOL©) Questionnaire Using Pooled Data from the Phase 3 BE HEARD I & II Trials of Bimekizumab in Hidradenitis Suppurativa., PMID:40172122
[Hidradenitis suppurativa]., PMID:40171910
Single-Injection Options for Administering a 320 mg Dose of Bimekizumab: 2 mL Safety Syringe and Auto-injector., PMID:40156698
Hidradenitis Suppurativa Symptom Daily Diary (HSSDD) and Questionnaire (HSSQ): Psychometric Validation and Interpretation Threshold Derivation Using Phase 3 Study Data., PMID:40153232
[Hot Topics 2024 and 2025 in dermatology]., PMID:40145186
An updated safety review of Hidradenitis Suppurativa treatment options., PMID:40125932
Bimekizumab for Psoriasis: Raising the Bar in Treatment., PMID:40116174
Short-Term Biologic Therapy for Guttate Psoriasis: Successful Treatment with Bimekizumab and Literature Review., PMID:40113443
Long-term maintenance of responses to bimekizumab treatment in moderate-to-severe psoriasis: A real-world comparison of Q4W versus Q8W dosing or bio-naïve versus bio-switched patients., PMID:40087893
Bimekizumab in the Treatment of Axial Spondyloarthritis and Psoriatic Arthritis: A New Kid on the Block., PMID:40076933
Efficacy and safety of biologics for hidradenitis suppurativa: A network meta-analysis of phase III trials., PMID:40062409
Severe Paradoxical Scalp Psoriasis Induced by Bimekizumab in a Young Multifailure Hidradenitis Suppurativa Patient., PMID:40016589
Efficacy and Safety of Interleukin-17 and Janus Kinase Inhibitors in Ankylosing Spondylitis: A Systematic Review and Network Meta-Analysis., PMID:40010328
Indirect Comparison Between Bimekizumab and Brodalumab for the Management of Moderate to Severe Psoriasis: A 36-Week Real-Life Study., PMID:39982649
A Case of Localised Immediate Hypersensitivity Reaction to Bimekizumab., PMID:39980236
Intra-class switch among interleukin-17 inhibitors for the treatment of plaque psoriasis: a single-center experience., PMID:39969045
Response to Sood et al's "Real-world experience of bimekizumab for plaque psoriasis in adult patients with prior exposure to interleukin-17 inhibitors: A 16-week multicenter retrospective review"., PMID:39956201
Response to Potestio et al., "Comment on Real-world experience of bimekizumab for plaque psoriasis in adult patients with prior exposure to interleukin-17 inhibitors: A 16-week multicenter retrospective review"., PMID:39955006
Bimekizumab for Hidradenitis Suppurativa: Pathophysiology and Promising Interventions., PMID:39946257
Tumor necrosis factor (TNF) inhibitors for psoriatic arthritis., PMID:39945386
Bimekizumab for the treatment of moderate to severe psoriasis: a real-world experience over 52 weeks from two Italian dermatology clinics., PMID:39925216
Bimekizumab: A novel FDA approved dual IL-17 A/F inhibitor for moderate to severe psoriasis., PMID:39912180
Real-World Experience of Bimekizumab for Plaque Psoriasis in Adult Patients with Prior Exposure to Interleukin-23 Inhibitors: A Multicenter Retrospective Study., PMID:39900871
Effect of Bimekizumab on Patient-Reported Outcomes and Work Productivity in Patients With Psoriatic Arthritis: 1-Year Results From 2 Phase III Studies., PMID:39892885
Long-term safety and sustained efficacy of bimekizumab in patients with ankylosing spondylitis (radiographic axial spondyloarthritis): 5-year results from BE AGILE (phase 2b) and its open-label extension., PMID:39890205
Hidradenitis Suppurativa Treatment During Pregnancy and Lactation: Navigating Challenges., PMID:39887706
Interleukin-17: A pleiotropic cytokine implicated in inflammatory, infectious, and malignant disorders., PMID:39875232