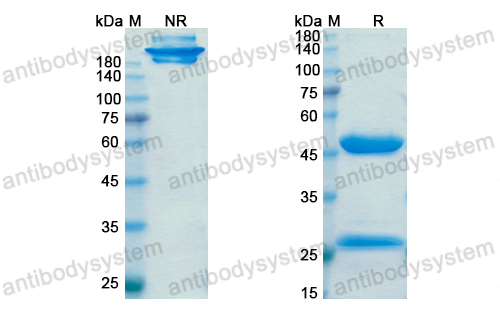
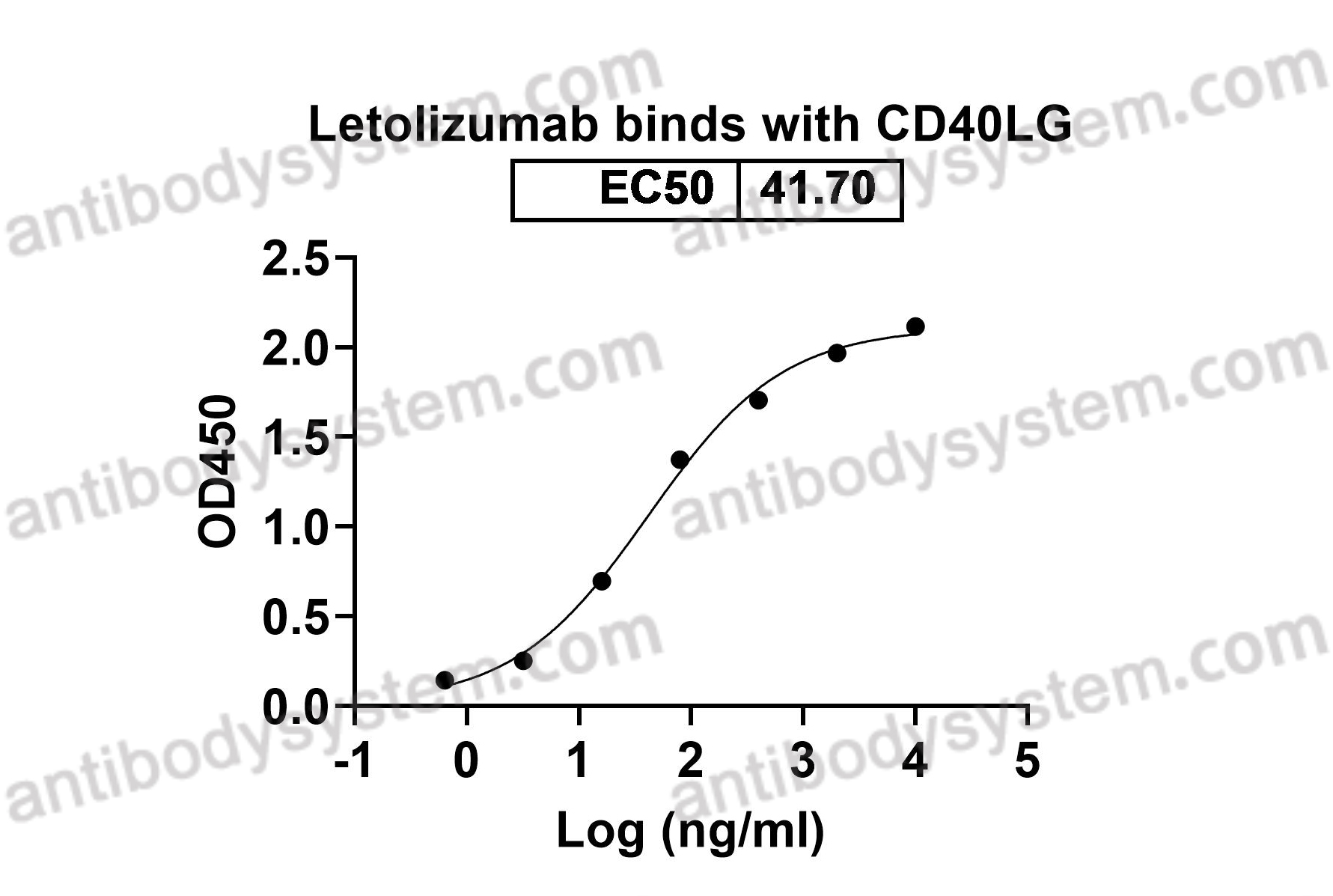
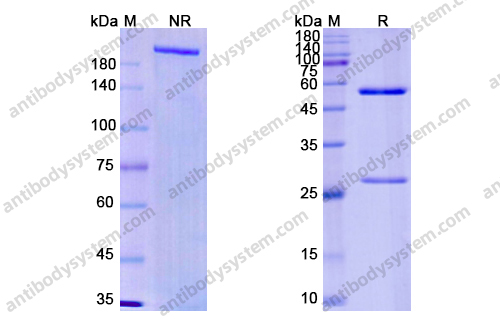
Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial, PMID: 31590988
Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis, PMID: 30242316
Immunotherapy in Small Cell Lung Cancer, PMID: 32899891
Immune checkpoint inhibitors for hepatocellular carcinoma, PMID: 31290997
Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial, PMID: 32271377
The role of immunotherapy in small cell lung cancer, PMID: 30637710
Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial, PMID: 33285097
Management of immune-related adverse events and kinetics of response with ipilimumab, PMID: 22614989
Antibodies to watch in 2019, PMID: 30516432
Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis, PMID: 30409774
Genetic basis for clinical response to CTLA-4 blockade in melanoma, PMID: 25409260
Immune checkpoint inhibitor-induced musculoskeletal manifestations, PMID: 32743706
Durvalumab for the treatment of non-small cell lung cancer, PMID: 31782989
[Classification and etiology of hyperthyroidism], PMID: 24779222
Immunotherapy of Hepatocellular Carcinoma: Facts and Hopes, PMID: 29138342
Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies, PMID: 29576375
Systemic Therapy for Hepatocellular Carcinoma: Latest Advances, PMID: 30380773
Combined Strategies with Poly (ADP-Ribose) Polymerase (PARP) Inhibitors for the Treatment of Ovarian Cancer: A Literature Review, PMID: 31374917
Immunotherapy in Bladder Cancer: Current Methods and Future Perspectives, PMID: 32392774
Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study, PMID: 32294530
Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma, PMID: 33046869
Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study, PMID: 32379280
Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma, PMID: 27816492
Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis, PMID: 29778737
Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma, PMID: 31954495
Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC, PMID: 29991499
Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3 + Regulatory T Cells (Tregs) in Human Cancers, PMID: 30054281
Immunotherapy for small-cell lung cancer: emerging evidence, PMID: 26882955
Trends in the treatment of advanced hepatocellular carcinoma: immune checkpoint blockade immunotherapy and related combination therapies, PMID: 31497341
Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis, PMID: 26337719
IMpower, CASPIAN, and more: exploring the optimal first-line immunotherapy for extensive-stage small cell lung cancer, PMID: 32503595
Prolonging Survival: The Role of Immune Checkpoint Inhibitors in the Treatment of Extensive-Stage Small Cell Lung Cancer, PMID: 32860288
Precision medicine phase II study evaluating the efficacy of a double immunotherapy by durvalumab and tremelimumab combined with olaparib in patients with solid cancers and carriers of homologous recombination repair genes mutation in response or stable after olaparib treatment, PMID: 32778095
Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial, PMID: 32971005
Safety and Efficacy of Durvalumab With or Without Tremelimumab in Patients With PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial, PMID: 30383184
Tremelimumab, PMID: 20698721
Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors, PMID: 30453170
Rationale and Outcomes for Neoadjuvant Immunotherapy in Urothelial Carcinoma of the Bladder, PMID: 33177001
ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer, PMID: 32201234
Recent advances and challenges of immune checkpoint inhibitors in immunotherapy of non-small cell lung cancer, PMID: 32450531
Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients, PMID: 32643323
Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): a biomarker-driven master protocol, PMID: 33125909
Safety and Efficacy of Durvalumab and Tremelimumab Alone or in Combination in Patients with Advanced Gastric and Gastroesophageal Junction Adenocarcinoma, PMID: 31676670
Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade, PMID: 32816849
Design and Rationale for a Phase III, Randomized, Placebo-controlled Trial of Durvalumab With or Without Tremelimumab After Concurrent Chemoradiotherapy for Patients With Limited-stage Small-cell Lung Cancer: The ADRIATIC Study, PMID: 31948903
Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: A systematic review, PMID: 32000444
Development of immunotherapy in bladder cancer: present and future on targeting PD(L)1 and CTLA-4 pathways, PMID: 29855698
Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis, PMID: 30716481
A Blood-based Assay for Assessment of Tumor Mutational Burden in First-line Metastatic NSCLC Treatment: Results from the MYSTIC Study, PMID: 33355200
Endocrine side effects induced by immune checkpoint inhibitors, PMID: 23471977
Neutrophil-Lymphocyte Ratio Predicts Overall Survival in Patients With HCC Treated With Durvalumab Plus Tremelimumab., PMID:40515751
Conversion surgery following severe cytokine release syndrome induced by immune checkpoint inhibitors doublet in advanced hepatocellular carcinoma., PMID:40500480
Editorial Comment on "Safety of Partial and Radical Nephrectomy for Complex Locally Advanced Renal Cell Carcinoma After Neo-Adjuvant Immune Checkpoint Inhibition - Analysis from a Phase 1b Trial (Durvalumab +/- Tremelimumab)"., PMID:40484289
Immune-mediated enterocolitis is associated with immune checkpoint inhibitors: A pharmacovigilance study from the FDA Adverse Event Reporting System (FAERS) database., PMID:40465755
Differential safety profiles of durvalumab monotherapy and durvalumab in combination with tremelimumab in adult patients with advanced cancers., PMID:40447320
Safety of Partial and Radical Nephrectomy for Complex Locally Advanced Renal Cell Carcinoma After Neo-Adjuvant Immune Checkpoint Inhibition: Analysis From a Phase 1b Trial (Durvalumab +/- Tremelimumab)., PMID:40441307
A novel approach to evaluate the therapeutic efficacy of durvalumab and tremelimumab combination therapy in hepatocellular carcinoma., PMID:40433908
Looking Toward the Future: Emerging Therapies for Hepatocellular Carcinoma., PMID:40416920
Multicenter phase Ib/II study of second-line durvalumab and tremelimumab in combination with paclitaxel in patients with biomarker-selected metastatic gastric cancer., PMID:40399487
Immune-mediated adverse events and overall survival with tremelimumab plus durvalumab and durvalumab monotherapy in unresectable HCC: HIMALAYA phase III randomized clinical trial., PMID:40384092
First-line durvalumab therapy alone or in combination with tremelimumab for metastatic head and neck squamous cell carcinoma: A cost-effectiveness analysis., PMID:40378108
Durvalumab with or without tremelimumab in combination with chemotherapy in first-line metastatic non-small-cell lung cancer: outcomes by tumor mutational burden in POSEIDON., PMID:40334315
Cost-Utility Analysis of Durvalumab and Tremelimumab Versus Best Supportive Care in Refractory Metastatic Colorectal Cancer., PMID:40320238
Antitumor effects and immune-mediated adverse events of durvalumab plus tremelimumab treatment for unresectable hepatocellular carcinoma., PMID:40318087
Peripheral blood CD4+/CD8+ T cell ratio may have potential for predicting the treatment response of durvalumab plus tremelimumab therapy (STRIDE) for unresectable hepatocellular carcinoma: Preliminary report., PMID:40317865
Neutrophil-to-lymphocyte ratio at the start of the second course of durvalumab plus tremelimumab therapy predicts therapeutic efficacy in patients with advanced hepatocellular carcinoma: A multicenter analysis., PMID:40317623
Evaluating two rechallenge strategies of immune checkpoint inhibitors: Durvalumab plus tremelimumab in advanced hepatocellular carcinoma., PMID:40317555
Multimodality treatment in synchronous oligometastatic NSCLC: Analysis of the ETOP CHESS trial., PMID:40311307
Tremelimumab plus durvalumab versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: a cost-effectiveness analysis from the US payer perspective., PMID:40306910
Pharmacovigilance study on the reporting frequency of atrial fibrillation with immune checkpoint inhibitors: insights from FDA Adverse Event Reporting System., PMID:40290514
Usefulness of the Early Increase of Peripheral Blood Lymphocyte Count in Predicting Clinical Outcomes for Patients with Advanced Hepatocellular Carcinoma Treated with Durvalumab Plus Tremelimumab., PMID:40282450
Prior Immune Checkpoint Inhibitor Treatment Is a Risk Factor for Treatment-Related Adverse Events in Unresectable Hepatocellular Carcinoma Treated With Durvalumab Plus Tremelimumab., PMID:40276064
A phase I clinical trial of radiation therapy, durvalumab and tremelimumab in recurrent gynecologic cancer., PMID:40273550
Translaminar Screw Fixation for Giant C1 Lateral Mass Metastasis From Hepatocellular Carcinoma., PMID:40271322
Treatment Decision-Making in Unresectable Hepatocellular Carcinoma: Importance of Understanding the Different Response Patterns between IO plus Anti-VEGF and IO plus IO Regimens., PMID:40255877
Cardiological adverse events in hepatocellular carcinoma patients receiving immunotherapy: Influence of comorbidities and clinical outcomes., PMID:40245453
Immune checkpoint inhibitor-induced dyshidrotic eczema following tremelimumab therapy for hepatocellular carcinoma., PMID:40224217
Five-year overall survival update from the HIMALAYA study of tremelimumab plus durvalumab in unresectable HCC., PMID:40222621
Immune check point inhibitors for ocular adnexal and periocular tumors., PMID:40213294
Effects of cannabinoids on immune checkpoint inhibitor response: CCTG pooled analysis of individual patient data., PMID:40184324
A Case of Advanced Non-Small Cell Lung Cancer With Hepatocellular Carcinoma-Like Features Responding to a Combination of Durvalumab, Tremelimumab, Carboplatin, and Nab-Paclitaxel., PMID:40161114
Mid-term Combination Immunotherapy and its Effect on the Albumin-Bilirubin Score in Unresectable Hepatocellular Carcinoma., PMID:40155043
Comparative effectiveness of immunotherapy versus lenvatinib in advanced hepatocellular carcinoma: A real-world analysis using target trial emulation., PMID:40153442
A phase 1 study of durvalumab as monotherapy or combined with tremelimumab with or without azacitidine in patients with myelodysplastic syndrome., PMID:40153010
Fractures Associated with Immune Checkpoint Inhibitors: A Disproportionality Analysis of the World Health Organization Pharmacovigilance Database., PMID:40143113
Prompt initiation of durvalumab and tremelimumab for unresectable hepatocellular carcinoma in patients with chronic active hepatitis B: a phase 2 clinical trial., PMID:40128285
Epigenetic therapy sensitizes anti-PD-1 refractory head and neck cancers to immunotherapy rechallenge., PMID:40091844
Phase 2 study of neoadjuvant durvalumab plus docetaxel, oxaliplatin, and S-1 with surgery and adjuvant durvalumab plus S-1 for resectable locally advanced gastric cancer., PMID:40081945
Mutational Landscape of Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma and Association with Immune Checkpoint Inhibitor Outcomes., PMID:40080442
Advances in Immunotherapy in Hepatocellular Carcinoma., PMID:40076561
Treatment outcomes of hepatectomy and systemic chemotherapy based on oncological resectability criteria for hepatocellular carcinoma., PMID:40046525
Neoadjuvant Chemotherapy with Dual Immune Checkpoint Inhibitors for Advanced-Stage Ovarian Cancer: Final Analysis of TRU-D Phase II Nonrandomized Clinical Trial., PMID:40043003
What can real-world data teach us about treating patients with unresectable hepatocellular carcinoma?, PMID:40042586
Higher Absolute Lymphocyte Counts and Lower Des-γ-Carboxyprothrombin Levels After Treatment Initiation Are Associated With the Clinical Efficacy of Tremelimumab Plus Durvalumab Combination Therapy for Hepatocellular Carcinoma., PMID:39989844
Multiomics analysis of immune correlatives in hepatocellular carcinoma patients treated with tremelimumab plus durvalumab., PMID:39965889
pH-dependent dissociation from CTLA-4 in early endosomes improves both safety and antitumor activity of anti-CTLA-4 antibodies., PMID:39964714
Bladder Preservation with Durvalumab plus Tremelimumab and Concurrent Radiotherapy in Patients with Localized Muscle-Invasive Bladder Cancer (IMMUNOPRESERVE): A Phase II Spanish Oncology GenitoUrinary Group Trial., PMID:39957401
Exacerbation of dipeptidyl peptidase-IV inhibitor-associated bullous pemphigoid by the immune checkpoint inhibitors durvalumab and tremelimumab., PMID:39953771
A clinical study of tremelimumab, alone or in combination with olaparib, for recurrent epithelial ovarian cancer., PMID:39951918
Self-Organizing Map-Based Assessment of Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors., PMID:39902026