Catalog No.
DHE14001
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappaorIGG2
Clonality
Monoclonal
Target
Hepatoma-associated antigen, BSG, HAb18G, 5F7, TCSF, OK blood group antigen, Collagenase stimulatory factor, CD147, Leukocyte activation antigen M6, Basigin, Extracellular matrix metalloproteinase inducer, EMMPRIN, Tumor cell-derived collagenase stimulatory factor
Concentration
1.8 mg/ml
Endotoxin level
Please contact with the lab for this information.
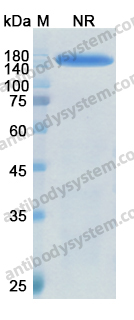
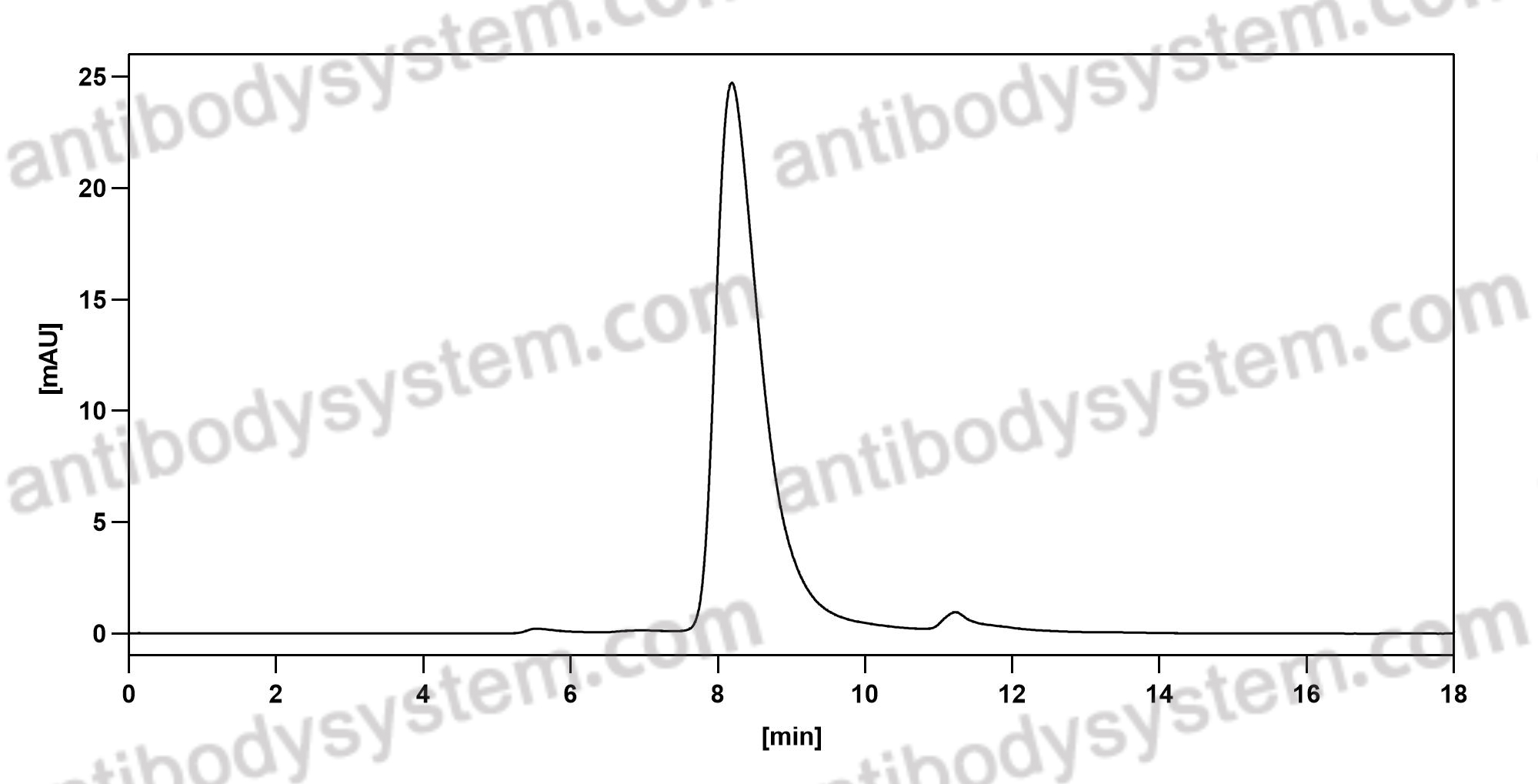
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P35613
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Clone ID
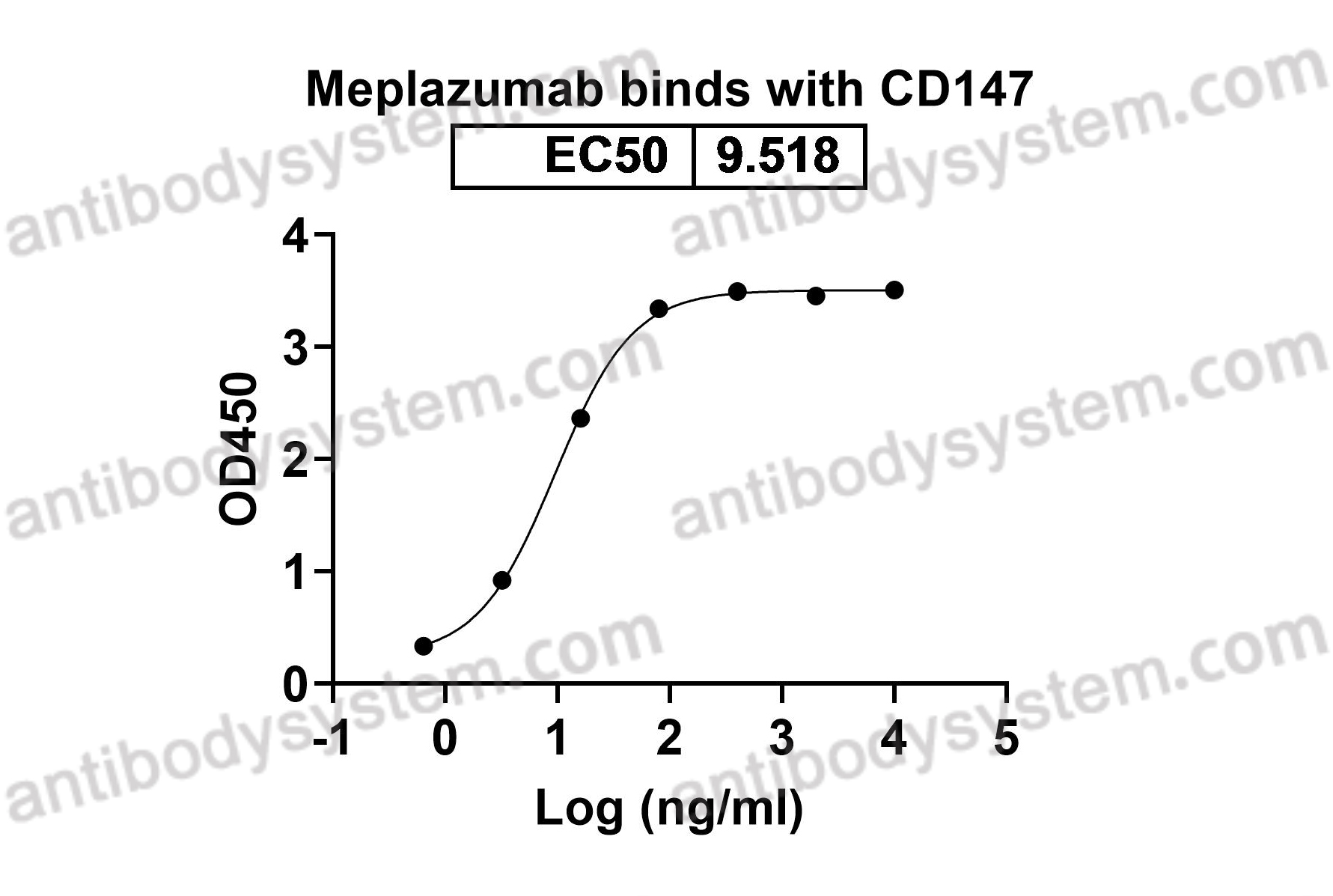
Meplazumab
Possible treatment and strategies for COVID-19: review and assessment, PMID: 33336780
FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy, PMID: 32717568
Nonclinical safety, tolerance and pharmacodynamics evaluation for meplazumab treating chloroquine-resistant Plasmodium falciparum, PMID: 33088688
CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells, PMID: 33277466
Tumor markers as an entry for SARS-CoV-2 infection?, PMID: 32738184
Safety and efficacy of meplazumab in healthy volunteers and COVID-19 patients: a randomized phase 1 and an exploratory phase 2 trial, PMID: 34001849
CD147 as an alternative binding site for the spike protein on the surface of SARS-CoV-2, PMID: 33336782
Meplazumab, a CD147 antibody, for severe COVID-19: a double-blind, randomized, placebo-controlled, phase 3 clinical trial., PMID:40222976
Comparative assessment of different anti-CD147/Basigin 2 antibodies as a potential therapeutic anticancer target by molecular modeling and dynamic simulation., PMID:38587771
Meplazumab in hospitalized adults with severe COVID-19 (DEFLECT): a multicenter, seamless phase 2/3, randomized, third-party double-blind clinical trial., PMID:36717539
Safety, Biodistribution, and Dosimetry Study of Meplazumab, a Potential COVID-19 Therapeutic Drug, with 131I-Labeling and SPECT Imaging., PMID:36668905
CD147 contributes to SARS-CoV-2-induced pulmonary fibrosis., PMID:36424379
CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target., PMID:34863742
CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma., PMID:34564690
Safety and efficacy of meplazumab in healthy volunteers and COVID-19 patients: a randomized phase 1 and an exploratory phase 2 trial., PMID:34001849
CD147 as an alternative binding site for the spike protein on the surface of SARS-CoV-2., PMID:33336782
Possible treatment and strategies for COVID-19: review and assessment., PMID:33336780
CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells., PMID:33277466
Nonclinical safety, tolerance and pharmacodynamics evaluation for meplazumab treating chloroquine-resistant Plasmodium falciparum., PMID:33088688
Tumor markers as an entry for SARS-CoV-2 infection?, PMID:32738184
FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy., PMID:32717568