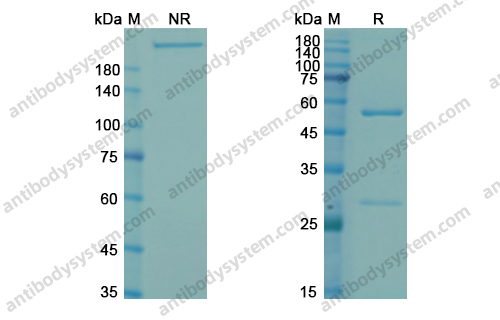
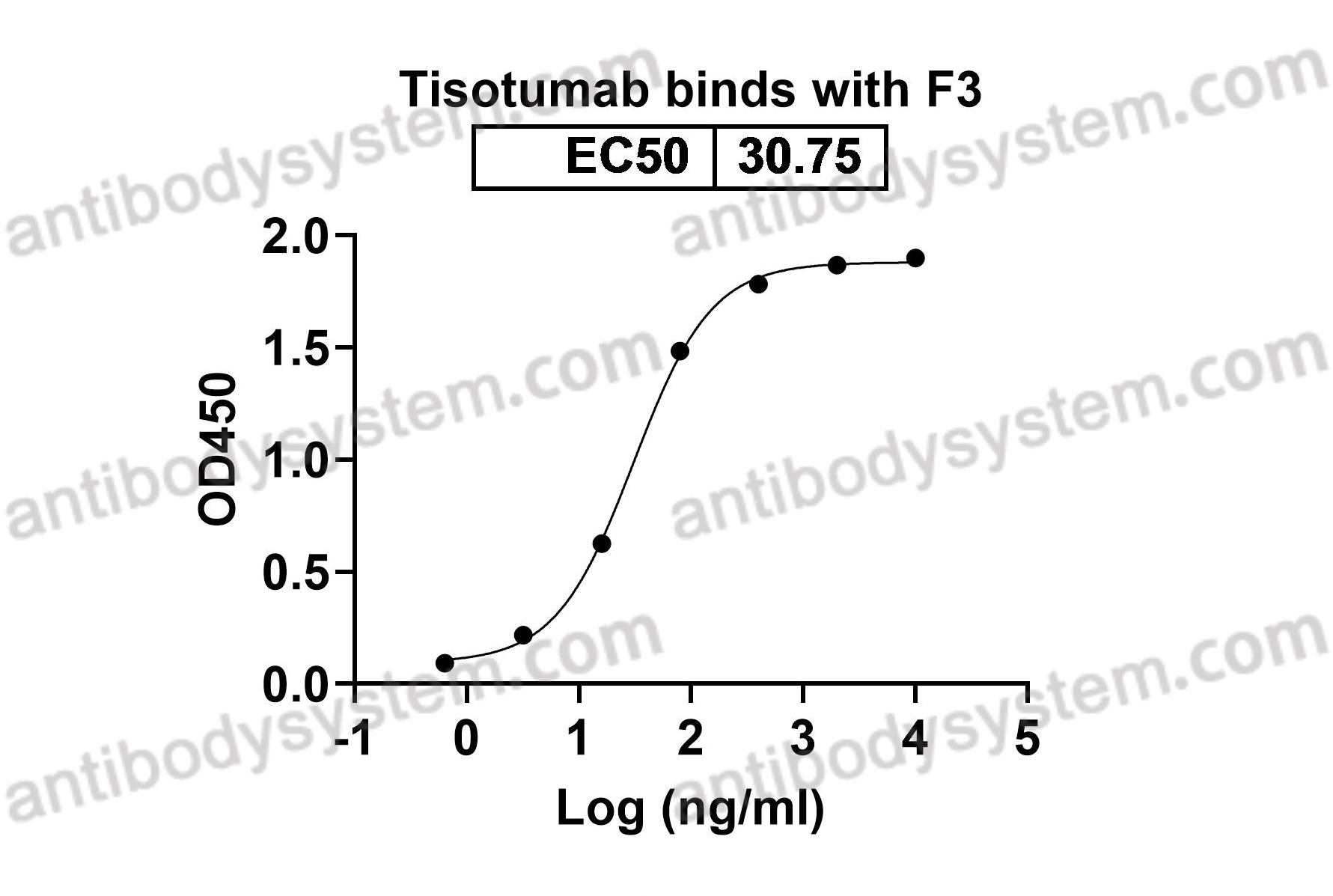
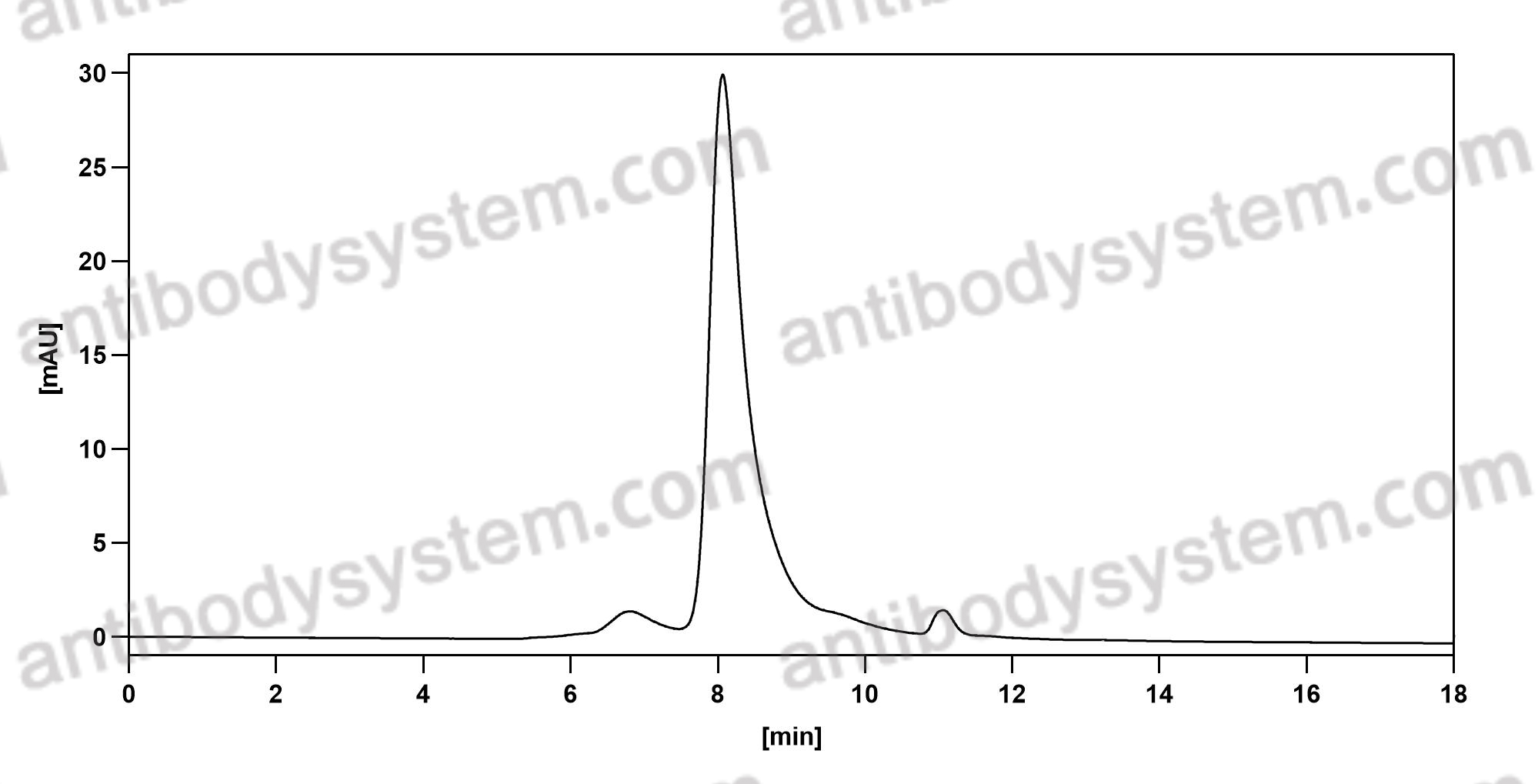
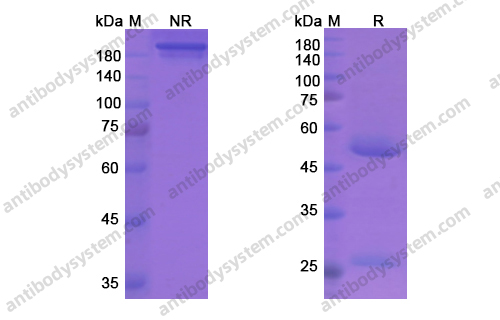
Catalog No.
DHD00601
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Tissue factor, CD142, F3, Thromboplastin, Coagulation factor III, TF
Concentration
9.33 mg/ml
Endotoxin level
Please contact with the lab for this information.
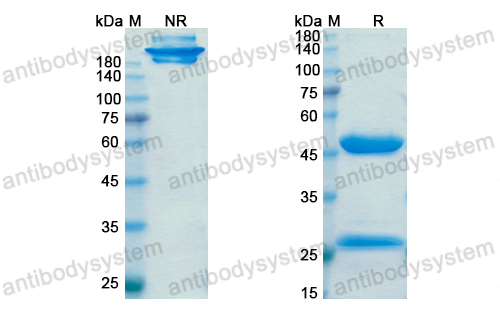
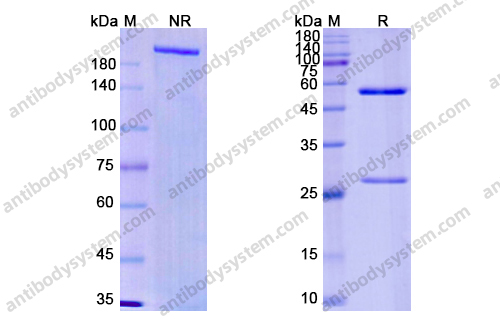
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P13726
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
100 mM Pro-Ac, 20 mM Arg, pH 5.0
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
HuMax-TF-ADC, TF-011-MMAE, HuMax-TF, CAS: 1418628-81-5
Clone ID
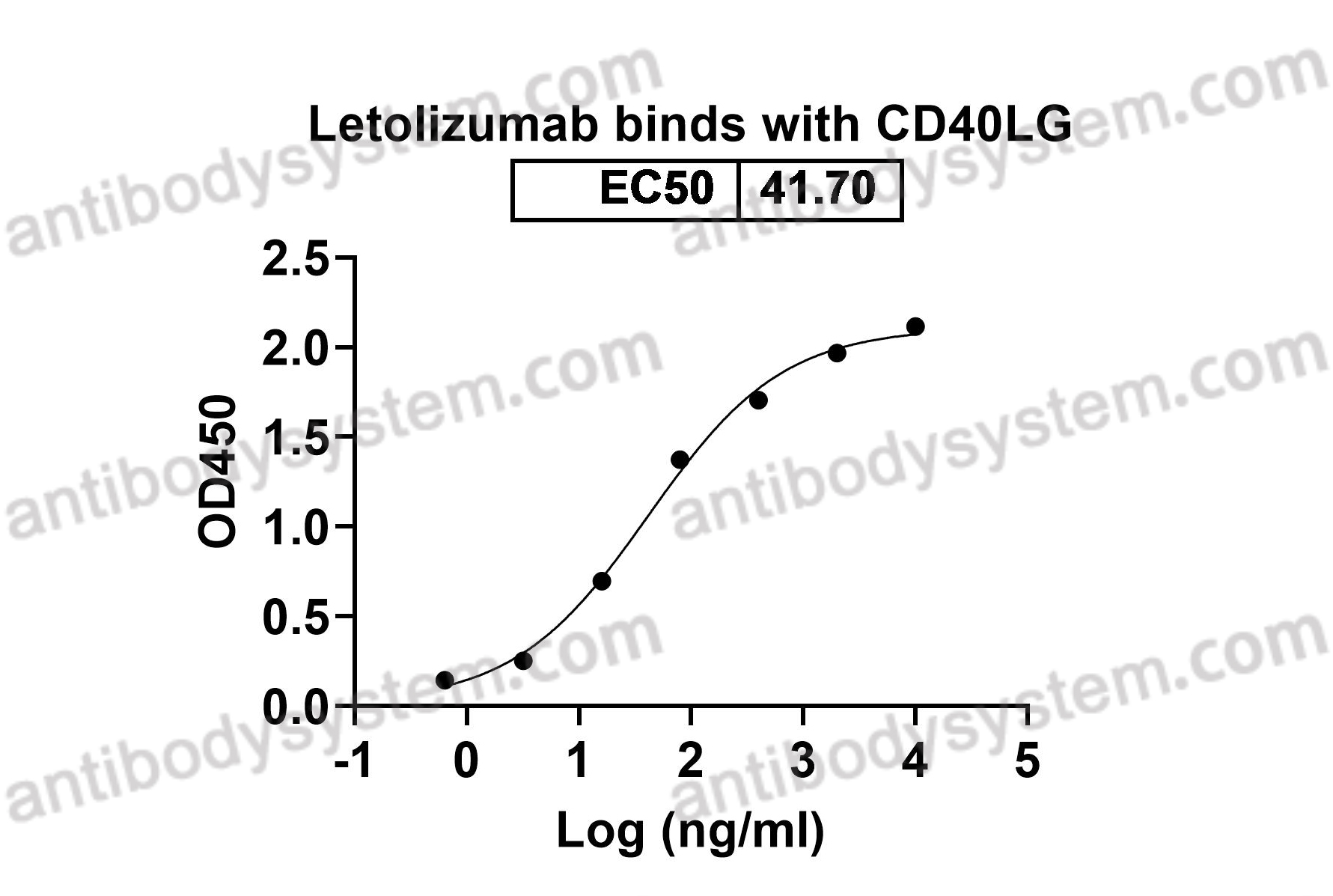
Tisotumab
Tisotumab Vedotin in Previously Treated Recurrent or Metastatic Cervical Cancer, PMID: 31796521
Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1-2 trial, PMID: 30745090
Tisotumab Vedotin Yields Responses as Second-Line Cervical Cancer Therapy, PMID: 33931438
Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study, PMID: 33845034
Treating Tissue Factor-Positive Cancers with Antibody-Drug Conjugates That Do Not Affect Blood Clotting, PMID: 30126944
Linoleic acid promotes TF expression through PPAR-α, which leads to tumor progression in primary pulmonary lymphoepithelioma-like carcinoma. [DHD00601]
[Pharmacological characteristics of tisotumab vedotin (recombinant) (TIVDAK® 40 mg Intravenous Solution) and clinical study results in recurrent or metastatic cervical cancer]., PMID:40518306
The efficacy and safety of Tisotumab vedotin in the treatment of recurrent/metastatic cervical cancer: a systematic review and meta-analysis of single-arm studies., PMID:40458792
Rapidly progressive pneumonitis days after receiving tisotumab vedotin: a new antibody-drug conjugate., PMID:40234078
PMDA regulatory update on approval and revision of the precautions for use of anticancer drugs; approval of amivantamab plus lazertinib for non-small cell lung cancer, durvalumab for small cell lung cancer, tislelizumab for esophageal cancer, tisotumab vedotin for cervical cancer, ivosidenib for leukemia, and venetoclax for lymphoma in Japan., PMID:40214878
Clinical applications of antibody drug conjugates for gynecologic malignancies: Review of available medicines and emerging therapeutics., PMID:40139025
Second-line antibody-drug conjugates for the treatment of metastatic human papillomavirus-independent gastric-type adenocarcinoma of cervix: The Singapore experience., PMID:40115377
F3 Expression Drives Sensitivity to the Antibody-Drug Conjugate Tisotumab Vedotin in Glioblastoma., PMID:40075681
Practical clinical management of ocular adverse events related to Antibody-Drug Conjugates in gynaecological malignancies., PMID:39970828
Ocular surface disease related to tisotumab vedotin-tftv., PMID:39877468
Tissue factor targeted near-infrared photoimmunotherapy: a versatile therapeutic approach for malignancies., PMID:39751657
Ocular adverse events associated with antibody-drug conjugates: a comprehensive pharmacovigilance analysis., PMID:39742259
Antibody-Drug Conjugates: The Toxicities and Adverse Effects That Emergency Physicians Must Know., PMID:39641680
Antibody-Drug Conjugates: A Start of a New Era in Gynecological Cancers., PMID:39590153
Tisotumab vedotin extravasation injury in a patient with recurrent cervical cancer., PMID:39431057
Safety and efficacy of tisotumab vedotin with cervical cancers: A systematic review and meta-analysis., PMID:39428336
Real-World Large Sample Assessment of Drug-related Dry Eye Risk: Based on the FDA Adverse Event Reporting System Database., PMID:39343068
Antibody drug conjugates in recurrent or metastatic cervical cancer: a focus on tisotumab vedotin state of art., PMID:39323928
Anti-tissue factor antibody conjugated with monomethyl auristatin E or deruxtecan in pancreatic cancer models., PMID:39322584
Pharmacovigilance study of the association between peripheral neuropathy and antibody-drug conjugates using the FDA adverse event reporting system., PMID:39271716
Discrepancy in PD-L1 expression between primary and metastatic tumors in two patients with recurrent cervical cancer., PMID:39252760
[Antibody-Drug Conjugates in Breast Cancer and Gynecologic Cancer]., PMID:39191683
Accuracy and Completeness of Large Language Models About Antibody-Drug Conjugates and Associated Ocular Adverse Effects., PMID:39110155
Tisotumab vedotin effective in recurrent cervical cancer., PMID:39039198
Tisotumab Vedotin as Second- or Third-Line Therapy for Recurrent Cervical Cancer., PMID:38959480
Tisotumab vedotin (Tivdak) for cervical cancer., PMID:38905529
Ocular toxicities associated with antibody drug conjugates., PMID:38814581
Exploring tisotumab vedotin in recurrent cervical cancer: A case series including an HPV-independent gastric type adenocarcinoma., PMID:38523623
Cost effectiveness of immunotherapy combination therapies for endometrial cancer., PMID:38449799
Recent Therapeutic Advances in Gynecologic Oncology: A Review., PMID:38398161
Cost-effectiveness of tisotumab vedotin as a second- or third-line therapy for cervical cancer., PMID:38330381
Acute keratoconjunctivitis associated with tisotumab vedotin-tftv for metastatic cervical cancer., PMID:38230392
Antibody-Drug Conjugates in Gynecologic Cancers., PMID:38172449
Assessing safety concerns of interstitial lung disease associated with antibody-drug conjugates: a real-world pharmacovigilance evaluation of the FDA adverse event reporting system., PMID:38100054
A review of the state of cervical cancer: updates from prevention to recurrent disease., PMID:37873756
An Antibody-Drug Conjugate Directed to Tissue Factor Shows Preclinical Antitumor Activity in Head and Neck Cancer as a Single Agent and in Combination with Chemoradiotherapy., PMID:37828725
New Paradigms in the Treatment of Cervical Cancer., PMID:37826852
SGN-B7H4V, an investigational vedotin ADC directed to the immune checkpoint ligand B7-H4, shows promising activity in preclinical models., PMID:37793853
Therapeutic Potential of Tisotumab Vedotin in the Treatment of Recurrent or Metastatic Cervical Cancer: A Short Report on the Emerging Data., PMID:37790898
Uncovering therapeutic opportunities in the clinical development of antibody-drug conjugates., PMID:37740463
Tisotumab Vedotin in Combination With Carboplatin, Pembrolizumab, or Bevacizumab in Recurrent or Metastatic Cervical Cancer: Results From the innovaTV 205/GOG-3024/ENGOT-cx8 Study., PMID:37651655
Sequential Targeted Therapy for Advanced, Metastatic, and Recurrent Cervical Cancer: A Cost-Effectiveness Analysis of the Patient Journey., PMID:37646470
Antibody-Drug Conjugates in Solid Tumor Oncology: An Effectiveness Payday with a Targeted Payload., PMID:37631374
Antibody-Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence., PMID:37568702
The abscopal effect of immune-radiation therapy in recurrent and metastatic cervical cancer: a narrative review., PMID:37539054
Exposure-safety and exposure-efficacy analyses for tisotumab vedotin for patients with locally advanced or metastatic solid tumors., PMID:37496366
Tissue factor (coagulation factor III): a potential double-edge molecule to be targeted and re-targeted toward cancer., PMID:37280670
Antibody-Drug Conjugates in Gynecologic Cancer., PMID:37229642
The evolving landscape of antibody-drug conjugates in gynecologic cancers., PMID:37023499
Linoleic acid drives pulmonary lymphoepithelioma-like carcinoma progression via PPAR-α/TF axis. [DHD00601]