Catalog No.
KAV00103
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant SARS-CoV-2 Spike Protein (BA.1) has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Anti-SARS-CoV-2 Spike Protein (BA.1) Human IgG present is bound by the immobilized protein. After washing away any unbound substances, a HRP-labeled Goat Anti-Human IgG antibody is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-SARS-CoV-2 Spike Protein (BA.1) Human IgG bound in the initial step. The color development is stopped and the intensity of the color is measured.
Specificity
Anti-SARS-CoV-2 Spike Protein (BA.1) Human IgG Antibody
Applications
Used for the quantitative determination of Anti-SARS-CoV-2 Spike Protein (BA.1) Human IgG concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
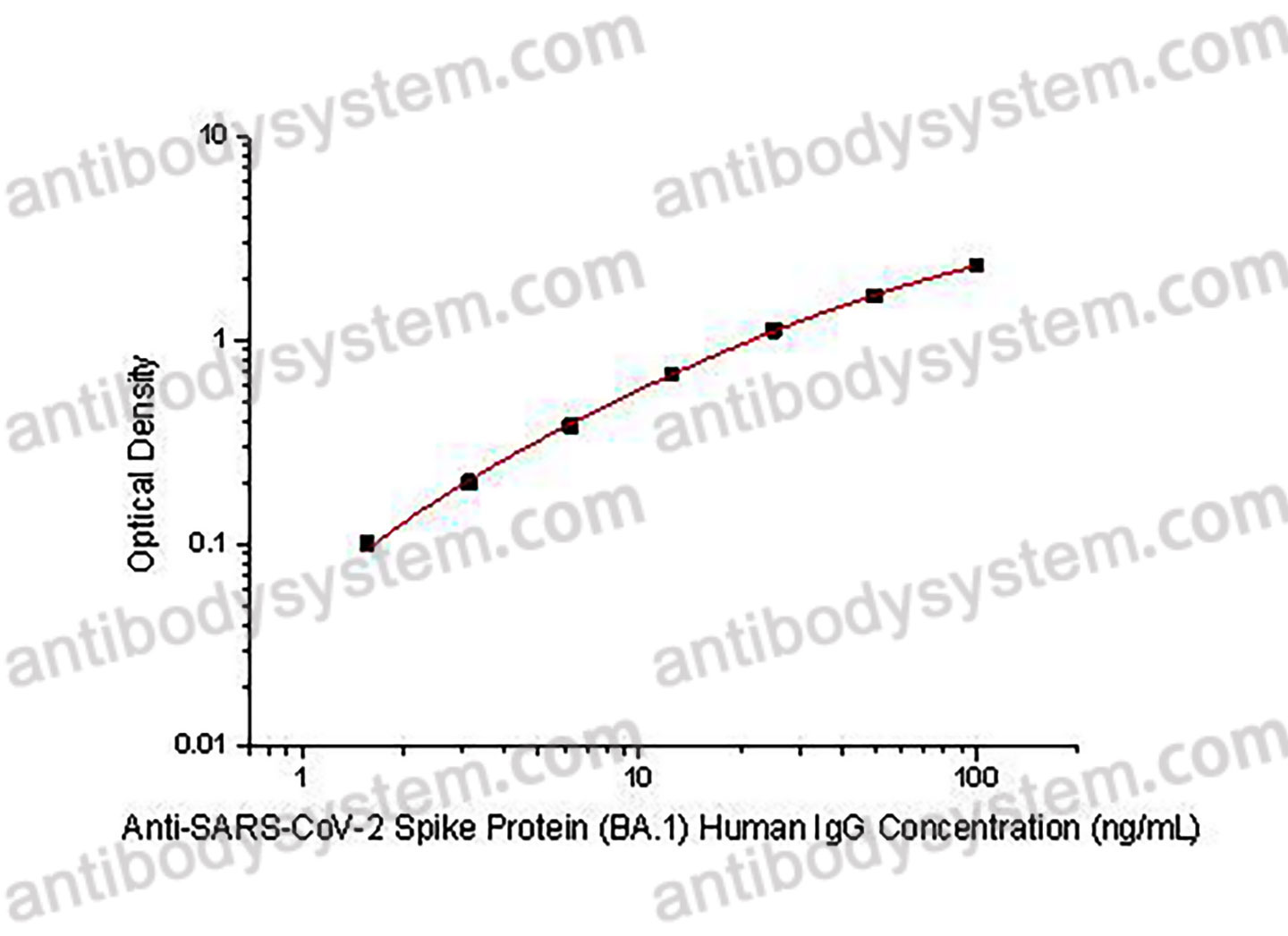
Range
1.56 - 100 ng/mL
Sensitivity
1.09 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
43.9
|
11.5
|
2.9
|
44.1
|
11.0
|
2.8
|
|
Standard deviation
|
3.2
|
0.6
|
0.1
|
2.3
|
0.5
|
0.2
|
|
CV (%)
|
7.2
|
5.0
|
3.3
|
5.2
|
4.8
|
5.5
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Characteristics of Kawasaki disease in children with a history of COVID-19., PMID:40517900
Covalent Functionalization of Layered Double Hydroxides to Generate Peptide-Based SARS-CoV-2 Nanovaccine., PMID:40508447
Detectable SARS-CoV-2 specific immune responses in recovered unvaccinated individuals 250 days post wild type infection., PMID:40498720
Immune age is correlated with decreased TCR clonal diversity and antibody response to SARS-CoV-2., PMID:40481111
Serum Levels of IL-21 and IL-27 Do not Reflect differential Avidity of Anti-SARS-CoV-2 IgG Antibodies in Symptomatic and Asymptomatic COVID-19 Patients., PMID:40471646
Systems serology-based comparison of humoral immune responses induced by liposome or aluminum hydroxide adjuvanted SARS-CoV-2 spike protein., PMID:40436929
Antibody Response Against SARS-CoV-2 Spike Protein in People with HIV After COVID-19 Vaccination., PMID:40432092
Impact of Vaccine-Elicited Anti-Spike IgG4 Antibodies on Fc-Effector Functions Against SARS-CoV-2., PMID:40431678
Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year., PMID:40431652
Evaluating SARS-CoV-2 T Cell Immunity in COVID-19-Naive Vaccinated Individuals with and Without Spike Protein IgG Antibodies., PMID:40430736
Development of a self-assembling multimeric Bann-RBD fusion protein in Pichia pastoris as a potential COVID-19 vaccine candidate., PMID:40425664
Comparative study of humoral and cellular immunity against SARS-CoV-2 induced by different COVID-19 vaccine types: Insights into protection against wildtype, Delta and JN.1 omicron strains., PMID:40408899
Evaluation of humoral and cellular immune responses in healthcare workers with varying levels of SARS-CoV-2 exposure: effects of CoronaVac vaccination followed by heterologous booster., PMID:40406109
The Combination of TLR4 and TLR9 Agonists with Self-Amplifying RNA Lipid Nanoparticles Leads to a More Powerful Immune Response Against SARS-CoV-2., PMID:40401447
Virus-specific antibody responses in multiple sclerosis patients treated with Ocrevus., PMID:40398376
Analysis of humoral and cellular immune activation up to 21 months after heterologous and homologous COVID-19 vaccination., PMID:40386778
Comprehensive analysis of human coronavirus antibody responses in ICU and non-ICU COVID-19 patients reveals IgG3 against SARS-CoV-2 spike protein as a key biomarker of disease severity., PMID:40359129
Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals., PMID:40358138
Mimicking immune complexes for efficient antibody responses., PMID:40356891
Impact of COVID-19 vaccination on symptoms and immune phenotypes in vaccine-naïve individuals with Long COVID., PMID:40346201
Epitope mapping of SARS-CoV-2 Spike protein using naturally-acquired immune responses to develop monoclonal antibodies., PMID:40346118
Identifying Neurological Autoantibodies in COVID-19: mGluR2 as a Marker of Immune Dysregulation During the Omicron Outbreak in China., PMID:40343769
Engineered bacteria as an orally administered anti-viral treatment and immunization system., PMID:40340796
Plasma SARS-CoV-2 nucleocapsid antigen levels are associated with lung infection and tissue-damage biomarkers., PMID:40339608
Comprehensive analysis of nasal IgA antibodies induced by intranasal administration of the SARS-CoV-2 spike protein., PMID:40338637
Using SARS-CoV-2 red cell kodecytes to assess vaccine-induced immune response to the conserved 1147-58 region of the spike protein in Indian blood donors: exploring the potential role of blood transfusion services in population surveillance., PMID:40335014
Temporal correlations between RBD-ACE2 blocking and binding antibodies to SARS-CoV-2 variants in CoronaVac-vaccinated individuals and their persistence in COVID-19 patients., PMID:40328892
Dynamics of SARS-CoV-2 Spike Receptor-Binding Domain-Targeted Specific Peripheral Memory B Cells in Patients With End-Stage Chronic Kidney Disease Undergoing Replacement Therapy Following COVID-19 Vaccination., PMID:40326950
Mucosal immune responses to SARS-CoV-2 infection and COVID-19 vaccination., PMID:40311214
SARS-CoV-2 S1 protein induces IgG-mediated platelet activation and is prevented by 1.8-cineole., PMID:40306177
A T7 autogene-mediated DNA vaccine platform for SARS-CoV-2: Overcoming DNA vaccine limitations with enhanced spike mRNA production., PMID:40287096
Characterization of Nanobody Binding to Distinct Regions of the SARS-CoV-2 Spike Protein by Flow Virometry., PMID:40285013
Evaluation of mRNA Transfection Reagents for mRNA Delivery and Vaccine Efficacy via Intramuscular Injection in Mice., PMID:40263125
Intranasal parainfluenza virus-vectored vaccine expressing SARS-CoV-2 spike protein of Delta or Omicron B.1.1.529 induces mucosal and systemic immunity and protects hamsters against homologous and heterologous challenge., PMID:40258004
Dual-screen-printed electrode platform for simultaneous detection of IgG and IgM antibodies using magnetic beads nanocomplexes., PMID:40250514
Nasal delivery of killed Bacillus subtilis spores protects against influenza, RSV and SARS-CoV-2., PMID:40242757
Modifying the glycosylation profile of SARS-CoV-2 spike-based subunit vaccines alters focusing of the humoral immune response in a mouse model., PMID:40217109
Spike specific IgG3 and nucleocapsid IgG response in serum serve as distinguishing immunological markers between SARS-CoV-2 infection and vaccination., PMID:40213555
Neutralizing antibodies to SARS-CoV-2 variants of concern: a pediatric surveillance study., PMID:40185990
Neutralizing antibody responses after a two-dose regimen with BNT162b2, CoronaVac or ChAdOx1-S in Brazil: Differential neutralization of SARS-CoV-2 omicron variants., PMID:40185297
Exploring TLR agonists as adjuvants for COVID-19 oral vaccines., PMID:40184639
Study protocol for a randomised controlled trial evaluating the efficacy of dietary modulation of probiotics on nutritional status and antibody response to SARS-CoV-2 in Indonesian adolescents: gut-lung axis (DIVINE)., PMID:40180370
Immunogenicity and safety of monovalent and bivalent SARS-CoV-2 variant adapted RBD-based protein booster vaccines in adults previously immunized with different vaccine platforms: A phase II/III, randomized clinical trial., PMID:40179522
Development of Thermostable and Immunogenic Block Copolymer Nanoparticles (BNPs) for mRNA Delivery., PMID:40163903
Virus-specific antibody responses in severe acute respiratory syndrome coronavirus 2-infected and vaccinated individuals., PMID:40157431
Optimizing Clinical Management of COVID-19: A Predictive Model for Unvaccinated Patients Admitted to ICU., PMID:40137715
Flow cytometric analysis of the SARS coronavirus 2 antibodies in human plasma., PMID:40133428
Rapid detection of systemic and mucosal antibody responses to COVID-19 infection or vaccination., PMID:40132457
Enhanced Humoral and Cellular Immune Responses Elicited by Salmonella Flagellin-Adjuvanted SARS-CoV-2 S1 Subunit Vaccine., PMID:40127244
Immunological assessment of NSFu1: A novel fusion molecule constructed from structural proteins of SARS-CoV-2 for improving COVID-19 antibody detection., PMID:40088274