Catalog No.
DHC02001
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
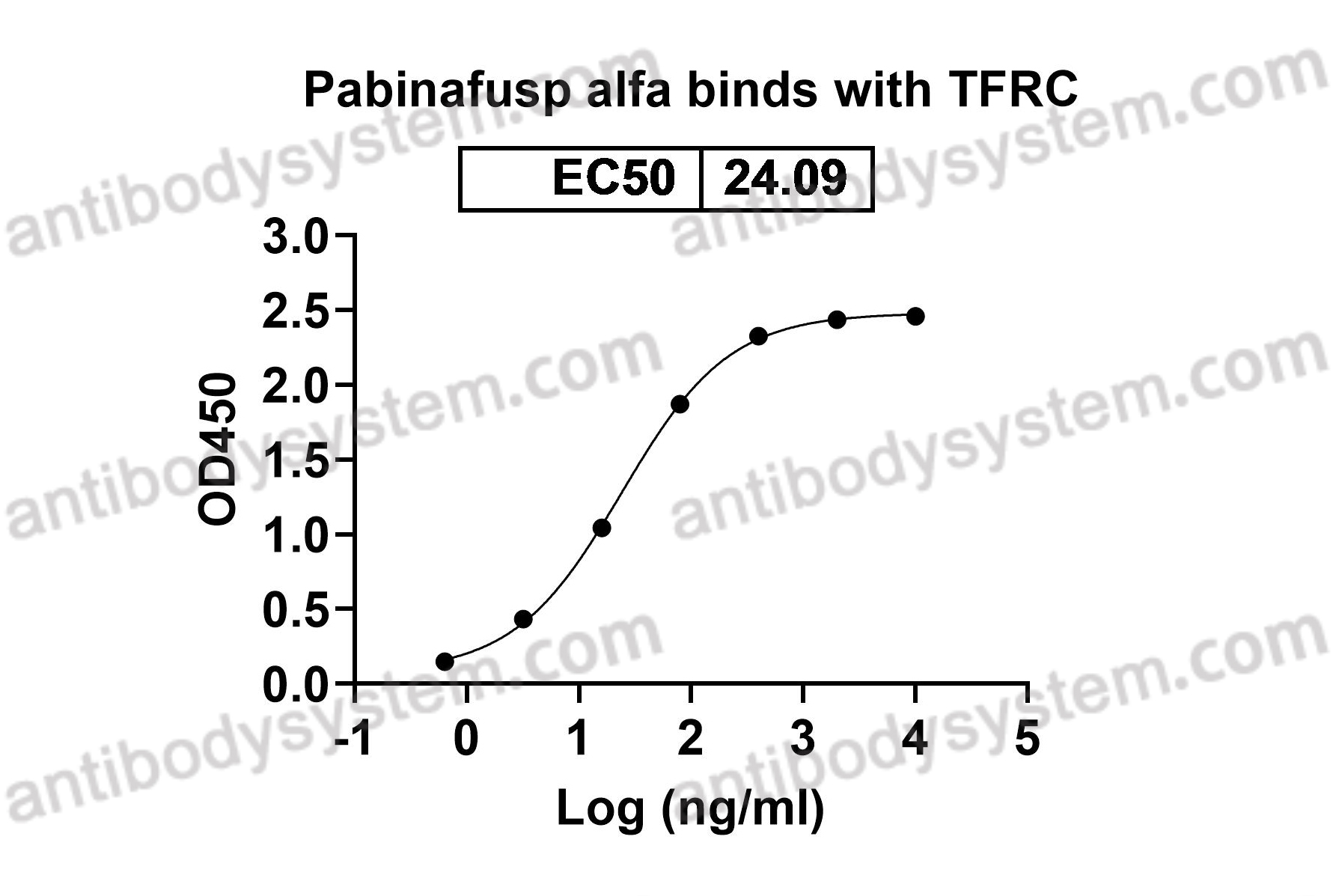
Target
Transferrin receptor protein 1, TfR1, T9, Trfr, p90, CD71, TFRC, TfR, sTfR, TR
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
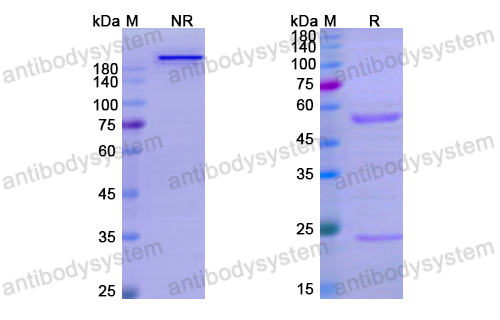
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P02786
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
JR-141, CAS: 2140211-48-7
Clone ID
Pabinafusp Alfa
A Phase 2/3 Trial of Pabinafusp Alfa, IDS Fused with Anti-Human Transferrin Receptor Antibody, Targeting Neurodegeneration in MPS-II, PMID: 33038326
Nonclinical safety evaluation of pabinafusp alfa, an anti-human transferrin receptor antibody and iduronate-2-sulfatase fusion protein, for the treatment of neuronopathic mucopolysaccharidosis type II, PMID: 33981582
Clearance of heparan sulfate in the brain prevents neurodegeneration and neurocognitive impairment in MPS II mice, PMID: 33508431
Iduronate-2-sulfatase fused with anti-hTfR antibody, pabinafusp alfa, for MPS-II: A phase 2 trial in Brazil, PMID: 33781915
Heparan sulfate in cerebrospinal fluid as a biomarker to assess disease severity and for treatment monitoring in patients with Mucopolysaccharidosis Type II: a position statement., PMID:39593190
Analysis of caregiver perspectives on patients with mucopolysaccharidosis II treated with pabinafusp alfa: results of qualitative interviews in Japan., PMID:38454486
Transferrin Receptor-Targeted Iduronate-2-sulfatase Penetrates the Blood-Retinal Barrier and Improves Retinopathy in Mucopolysaccharidosis II Mice., PMID:37860991
Treatment of Neuronopathic Mucopolysaccharidoses with Blood-Brain Barrier-Crossing Enzymes: Clinical Application of Receptor-Mediated Transcytosis., PMID:35745811
Dose-dependent effects of a brain-penetrating iduronate-2-sulfatase on neurobehavioral impairments in mucopolysaccharidosis II mice., PMID:35662814
Drug delivery for neuronopathic lysosomal storage diseases: evolving roles of the blood brain barrier and cerebrospinal fluid., PMID:35088290
Antibodies to watch in 2022., PMID:35030985
[Pharmacological property, mechanism of action and clinical study results of Pabinafusp Alfa (Genetical Recombination) (IZCARGO® I.V. Infusion 10 mg) as the therapeutic for Mucopolysaccharidosis type-II (Hunter syndrome)]., PMID:34980815
Divergent developmental trajectories in two siblings with neuropathic mucopolysaccharidosis type II (Hunter syndrome) receiving conventional and novel enzyme replacement therapies: A case report., PMID:34765392
Enzyme Replacement Therapy with Pabinafusp Alfa for Neuronopathic Mucopolysaccharidosis II: An Integrated Analysis of Preclinical and Clinical Data., PMID:34681597
Nonclinical safety evaluation of pabinafusp alfa, an anti-human transferrin receptor antibody and iduronate-2-sulfatase fusion protein, for the treatment of neuronopathic mucopolysaccharidosis type II., PMID:33981582
Iduronate-2-sulfatase fused with anti-hTfR antibody, pabinafusp alfa, for MPS-II: A phase 2 trial in Brazil., PMID:33781915
Clearance of heparan sulfate in the brain prevents neurodegeneration and neurocognitive impairment in MPS II mice., PMID:33508431
A Phase 2/3 Trial of Pabinafusp Alfa, IDS Fused with Anti-Human Transferrin Receptor Antibody, Targeting Neurodegeneration in MPS-II., PMID:33038326