Catalog No.
PHB86202
Species reactivity
Human
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
E. coli - derived recombinant Human F8/Coagulation factor VIII (Val2144-Tyr2351).
Tested applications
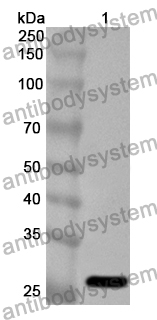
ELISA: 1:4000-1:8000, IHC: 1:50-1:100, WB: 1:1000-1:4000
Target
AHF, Procoagulant component, Coagulation factor VIII, Antihemophilic factor, F8C, F8
Purification
Purified by antigen affinity column.
Accession
P00451
Applications
ELISA, IHC, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Generation of a Severe Hemophilia A Humanized Mouse Model Capable of Inducing an Anti-FVIII Immune Response., PMID:40300593
A scan of pleiotropic immune mediated disease genes identifies novel determinants of baseline FVIII inhibitor status in hemophilia A., PMID:40263602
An engineered Treg selective immunocytokine induces sustained immune modulation in a preclinical model of hemophilia A., PMID:40056981
A randomized, two-armed, double-blind, single-dose, cross-over, bioequivalence clinical trial to compare pharmacokinetic parameters and safety of recombinant human factor VIII with Fc fusion produced by AryoGen Pharmed Company versus Elocta® (reference product) in previously treated patients with severe haemophilia A., PMID:39934428
Tolerance to factor VIII in the era of nonfactor therapies: immunologic perspectives and a systematic review of the literature., PMID:39800259
Accurate evaluation of factor VIII activity of efanesoctocog alfa in the presence of emicizumab., PMID:39798924
FVIII peptides presented on HLA-DP and identification of an A3 domain peptide binding with high affinity to the commonly expressed HLA-DP4., PMID:39665218
Verification and Implementation of a Bovine Chromogenic Factor VIII Assay for Hemophilia A Patients on Emicizumab Therapy., PMID:39662012
Exploring red blood cells as an antigen delivery system to modulate the immune response towards FVIII in hemophilia A., PMID:39617188
Acquired hemophilia A: a narrative review and management approach in the emicizumab era., PMID:39536818
Persistent splenic-derived IgMs preferentially recognize factor VIII A2 and C2 domain epitopes but do not alter antibody production., PMID:39476969
Comprehensive evaluation of anti-emicizumab antibodies in acquired hemophilia A: a detailed case study and methodological evaluation., PMID:39401737
CHIEF: A retrospective self-control study of children with severe hemophilia A without inhibitors comparing emicizumab to FVIII prophylaxis., PMID:39367598
Lack of factor VIII detection in humans and dogs with an intron 22 inversion challenges hypothesis regarding inhibitor risk., PMID:39233012
Large deletions and small insertions and deletions in the factor VIII gene predict unfavorable immune tolerance induction outcome in people with severe hemophilia A and high-responding inhibitors., PMID:39186847
Coagulation factor VIII: biological basis of emerging hemophilia A therapies., PMID:39088776
Recent Advances in Gene Therapy for Hemophilia: Projecting the Perspectives., PMID:39062568
Acid Treatment of FVIII-Containing Plasma Samples Unmasks a Broad Spectrum of FVIII-Specific Antibodies in ELISA., PMID:39009010
Development of a rapid and fully automated factor VIII inhibitor assay, insensitive to emicizumab, and a lowest level of quantification of 0.2 BU/mL., PMID:38992344
Emicizumab versus immunosuppressive therapy for the management of acquired hemophilia A., PMID:38936699
Structural basis for inhibition of coagulation factor VIII reveals a shared antigenic hotspot on the C1 domain., PMID:38849084
Simoctocog alfa (Nuwiq®) in children: early steps in life's journey for people with severe hemophilia A., PMID:38737006
Application of machine learning approaches for predicting hemophilia A severity., PMID:38718927
Ultra-Long factor VIII: a major step forward toward a hemophilia-free mind., PMID:38679336
Comprehensive domain-specific analysis and immunoglobulin G profiling of anti-factor VIII antibodies using a bead-based multiplex immunoassay., PMID:38453023
Comparison of thrombotic adverse events in patients treated with factor VIII products and emicizumab using the 2018-2022 US Food and Drug Administration Adverse Event Reporting System data., PMID:38395359
A Scan of Pleiotropic Immune Mediated Disease Genes Identifies Novel Determinants of Baseline FVIII Inhibitor Status in Hemophilia-A., PMID:37886476
A machine learning approach for identifying variables associated with risk of developing neutralizing antidrug antibodies to factor VIII., PMID:37251488
Bacterial Production of Recombinant Coagulation Factor VIII Domains., PMID:37109652
Race, ethnicity, F8 variants, and inhibitor risk: analysis of the "My Life Our Future" hemophilia A database., PMID:36696179
Anti-FVIII antibodies in Black and White hemophilia A subjects: do F8 haplotypes play a role?, PMID:36459498
Preimplantation genetic testing (PGT) for hemophilia A: Experience from one center., PMID:36427965
Translational readthrough at F8 nonsense variants in the factor VIII B domain contributes to residual expression and lowers inhibitor association., PMID:35924581
Genome-Wide Association Study and Gene-Based Analysis of Participants With Hemophilia A and Inhibitors in the My Life, Our Future Research Repository., PMID:35814780
Clinical conditions and risk factors for inhibitor-development in patients with haemophilia: A decade-long prospective cohort study in Japan, J-HIS2 (Japan Hemophilia Inhibitor Study 2)., PMID:35689832
Neutralizing Antibodies Against Factor VIII Can Occur Through a Non-Germinal Center Pathway., PMID:35634288
Revealing and IgG4 analysis to factor VIII in haemophilia-A patients with and without inhibitors., PMID:34949528
Serum TNF-α Level as a Possible Predictor of Inhibitor Levels in Severe Hemophilia A., PMID:34778454
Real-world experience on the tolerability and safety of emicizumab prophylaxis in paediatric patients with severe haemophilia A with and without FVIII inhibitors., PMID:34628693
An epileptic seizure and haemorrhage into the ventricular system of the brain as the first manifestations of acquired haemophilia A - Case report., PMID:34558281
Antigen-specific immunotherapy with apitopes suppresses generation of FVIII inhibitor antibodies in HLA-transgenic mice., PMID:34529764
Haemophilia: factoring in new therapies., PMID:34322873
Structure of Blood Coagulation Factor VIII in Complex With an Anti-C2 Domain Non-Classical, Pathogenic Antibody Inhibitor., PMID:34177966
Haemophilia., PMID:34168126
Protein residue network analysis reveals fundamental properties of the human coagulation factor VIII., PMID:34135429
Prediction of hemophilia A severity using a small-input machine-learning framework., PMID:34035274
Nonhuman glycans can regulate anti-factor VIII antibody formation in mice., PMID:34019619
Performance of a clinical risk prediction model for inhibitor formation in severe haemophilia A., PMID:33988289
Characterisation and application of recombinant FVIII-neutralising antibodies from haemophilia A inhibitor patients., PMID:33973229
The effectiveness and value of emicizumab and valoctocogene roxaparvovec for the management of hemophilia A without inhibitors., PMID:33908280