Catalog No.
KAD12601
Description
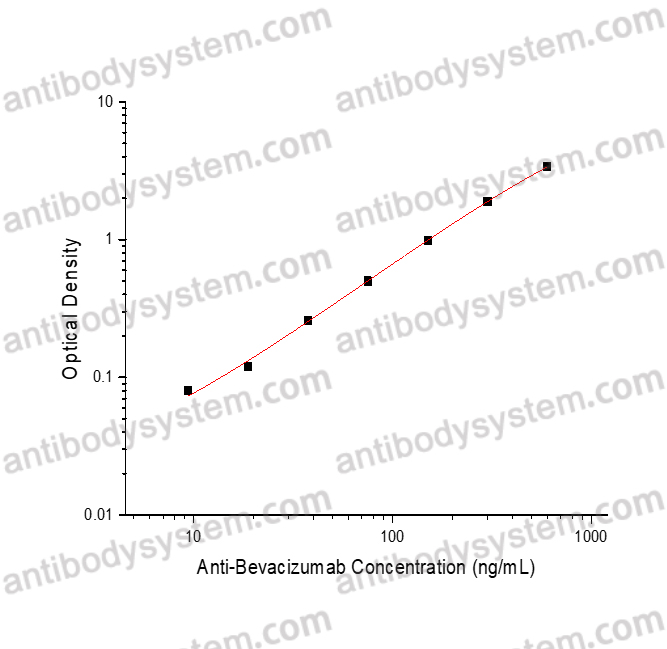
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Bevacizumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Bevacizumab will be captured by immobilized Bevacizumab. After washing away any unbound substances, a biotin-labeled Bevacizumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Bevacizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Bevacizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
9.38 - 600 ng/mL
Sensitivity
5.78 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
290.2
|
71.3
|
15.2
|
294.6
|
72.8
|
15.5
|
|
Standard deviation
|
8.9
|
2.4
|
1.3
|
6.8
|
2.3
|
1.7
|
|
CV (%)
|
3.1
|
3.4
|
8.7
|
2.3
|
3.2
|
11.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
12-IgG1,F(ab)-12 IgG1,Fab-12 IgG1,rhuMAb-VEGF, ABP 215,CAS: 216974-75-3
Bevacizumab-Conjugated Curcumin Nanoparticles Promote Cytotoxicity and Apoptosis in Human Malignant Oral Keratinocytes In Vitro., PMID:40514017
Predictive value of BRCA1/RAD51C methylation in HGSOC - An ancillary study of the PAOLA-1/ENGOT-ov25 phase 3 trial., PMID:40513287
Increased Hemorrhage During Excision of Bevacizumab-Treated NF2-Related Vestibular Schwannomas., PMID:40511882
Advances in Molecular Imaging of VEGFRs: Innovations in Imaging and Therapeutics., PMID:40508182
Real-World Experiences Using Atezolizumab + Bevacizumab for the Treatment of Unresectable Hepatocellular Carcinoma: A Multicenter Study., PMID:40507295
Revised European Society of Endocrinology Clinical Practice Guideline for the management of aggressive pituitary tumours and pituitary carcinomas., PMID:40506054
Advances in drug treatments for male patients with prolactinomas., PMID:40505642
The use of Bevacizumab in the treatment of brain arteriovenous malformations: a systematic review., PMID:40504282
Clinical and prognostic characteristics of metastatic colorectal cancer with minor RAS mutations., PMID:40503036
Mirvetuximab soravtansine for the treatment of epithelial ovarian, fallopian tube, or primary peritoneal cancer., PMID:40501444
A Neovascularization and EGFR Dual-Targeting Gold Nanotheranostic System for Non-Small Cell Lung Cancer Treatment., PMID:40500941
Response to the comment on our manuscript "Effect of systemic FOLFOXIRI plus bevacizumab treatment of colorectal peritoneal metastasis on local and systemic immune cells"., PMID:40500641
Targeting the ST3 beta-galactoside alpha-2,3-sialyltransferase 1 (ST3Gal1) as a potential therapeutic strategy to overcome anti-VEGF resistance in endometrial cancer., PMID:40497576
Obesity paradox role in the immunosuppressive treatment of hepatocellular carcinoma., PMID:40497086
Adoptive cellular immunotherapy combined with chemotherapy versus chemotherapy alone in Chinese patients with metastatic colorectal cancer: a cost-effectiveness analysis to inform drug pricing., PMID:40496606
Bevacizumab in recurrent epithelial ovarian cancer: real-world experience from a tertiary cancer hospital in India., PMID:40496305
Antineovascular Effects of Coenzyme Q10 and Vitamin E Combination in Corneal Neovascularization: A Pilot Study., PMID:40495727
Atezolizumab plus bevacizumab versus Lenvatinib for patients with Barcelona clinic liver cancer stage B (BCLC-B) hepatocellular carcinoma (HCC): A real-world population., PMID:40494131
Whole Body-MRI assessment of peripheral lesions in patients with NF2-related schwannomatosis on systemic bevacizumab., PMID:40493293
Children with medulloblastoma treated with modified ACNS0821 temozolomide, irinotecan, and bevacizumab: The Seattle Children's Hospital experience., PMID:40487589
Lenvatinib versus bevacizumab when combined with PD-1/L1 inhibitor and hepatic arterial infusion chemotherapy in unresectable hepatocellular carcinoma., PMID:40486515
Response to 'Comment on: 'Comparing safety and efficacy of Bevacizumab, Ranibizumab and Ranibizumab biosimilar in Retinopathy of prematurity"., PMID:40483306
Personalized bevacizumab dosing in metastatic colorectal cancer: Statistical considerations for a phase III randomized trial., PMID:40483175
Multimodal nanoparticles co-delivering bevacizumab and dichloroacetate for dual targeting of neoangiogenesis and hyperglycolysis in glioblastoma treatment., PMID:40482922
Revisiting the Role of Intravitreal Triamcinolone in Diabetic Macular Edema: 12-Month Outcomes after Bevacizumab Failure., PMID:40481980
Anti-VEGFs for Diabetic Macular Oedema: Analysis of Efficacy, Safety, and Cost of More Durable Therapies from a Dutch Societal Perspective., PMID:40481910
Characteristics and outcomes of primary and secondary resistance to immune checkpoint inhibitors in hepatocellular carcinoma., PMID:40481877
Intravitreal anti-vascular endothelial growth factor injections and risks of stroke in patients with neovascular age-related macular degeneration-A registry-based cohort study., PMID:40481786
Efficacy of Hepatic Artery Infusion Chemotherapy with Bevacizumab and Sintilimab in Advanced Hepatocellular Carcinoma: A Case Report., PMID:40481624
Safety and efficacy of systemic chemotherapy plus PD-1 inhibitor in combination with intravenous or intraperitoneal bevacizumab in gastric cancer with peritoneal metastasis., PMID:40481442
Validation of a 15-Gene Prognostic Signature in Metastatic Clear Cell Renal Cell Carcinoma., PMID:40479621
A case report of small cell neuroendocrine carcinoma of the ovary and review of the literature., PMID:40475766
Bevacizumab in ovarian cancer therapy: current advances, clinical challenges, and emerging strategies., PMID:40474872
Relacorilant and nab-paclitaxel in patients with platinum-resistant ovarian cancer (ROSELLA): an open-label, randomised, controlled, phase 3 trial., PMID:40473448
Clinical characteristics and treatment response of treatment requiring retinopathy of prematurity (ROP) in Big Premature Infants in Turkiye: BIG-ROP Study Group Report No 2 (BIG-ROP STUDY)., PMID:40473274
Transarterial chemoembolization combined with intra-arterial infusion of sintilimab and bevacizumab for advanced hepatocellular carcinoma: a phase 2 study., PMID:40472923
Design and characterization of intravitreal bevacizumab-loaded PLGA nanoparticles: pharmacokinetic and biodistribution impact., PMID:40471504
Complete Response of Hepatocellular Carcinoma With Inferior Vena Cava Tumor Thrombus and Right Atrium Involvement to Combined Radiotherapy and Immunotherapy: A Case Report., PMID:40470485
Refractory Diarrhea Related to EPHB4 Mutation in a Patient With Capillary Malformation-Arteriovenous Type 2 Syndrome., PMID:40469463
PD-1/PD-L1 inhibitors plus bevacizumab plus chemotherapy versus PD-1/PD-L1 inhibitors plus chemotherapy for advanced non-small cell lung cancer: a phase 3 RCT based meta-analysis., PMID:40469183
Atezolizumab plus paclitaxel and bevacizumab as first-line treatment of advanced triple-negative breast cancer: the ATRACTIB phase 2 trial., PMID:40467896
EFFICACY AND PROGNOSIS OF ANTI-VEGF AGENTS COMBINED WITH PANRETINAL PHOTOCOAGULATION IN DIABETIC RETINOPATHY: A CLINICAL OBSERVATIONAL STUDY., PMID:40466685
Dostarlimab and niraparib in primary advanced ovarian cancer., PMID:40461381
Reply to Comment on "Retinal vascularization rate predicts retinopathy of prematurity and remains unaffected by low-dose bevacizumab treatment"., PMID:40460926
Effect of antineoplastic drug therapies on carcinoma and aggressive pituitary tumors: a systematic review and meta-analysis., PMID:40457103
High CD36 expression in the tumor microenvironmental vasculature correlates with unfavorable overall survival in high grade serous ovarian cancer., PMID:40456794
S100B induces angiogenesis via the clathrin/FOXO1/β-catenin signaling pathway and contributes to Bevacizumab resistance in epithelial ovarian cancer., PMID:40456442
Association between intravitreal anti-vascular endothelial growth factor agents and hypertension: a meta-analysis., PMID:40456283
Anti-VEGF Agents Clearance Through the Aqueous Outflow Pathway in a Rat Model., PMID:40455045
Several Common Genetic Variations Associate With Functional or Anatomic Effects of Anti-VEGF Treatment in Conditions With Macular Edema., PMID:40455044