Catalog No.
KAB94403
Description
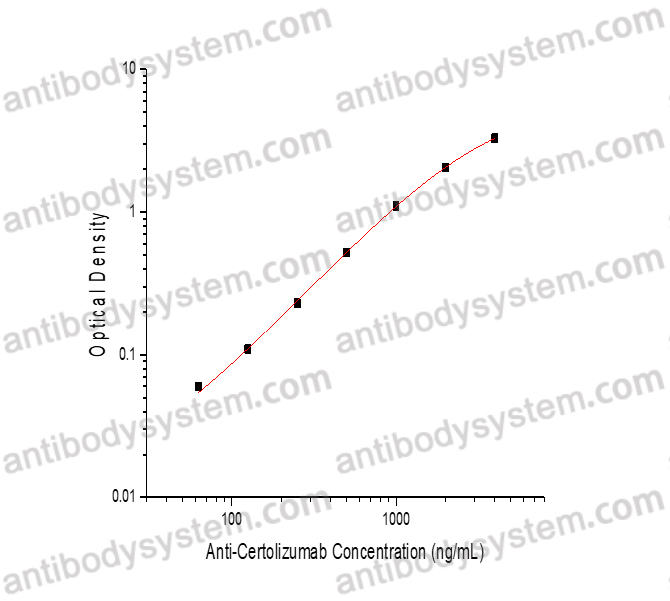
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Certolizumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Certolizumab will be captured by immobilized Certolizumab. After washing away any unbound substances, a biotin-labeled Certolizumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Certolizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Certolizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
62.5 - 4,000 ng/mL
Sensitivity
8.11 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1879.7
|
407.3
|
94.0
|
1841.5
|
319.0
|
70.6
|
|
Standard deviation
|
82.6
|
18.9
|
9.3
|
99.4
|
15.8
|
6.9
|
|
CV (%)
|
4.4
|
4.6
|
9.9
|
5.4
|
4.9
|
9.7
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CDP870,PHA-738144, Certolizumab pegol,CAS: 428863-50-7
Certolizumab pegol to prevent adverse pregnancy outcomes in patients with antiphospholipid syndrome and lupus anticoagulant (IMPACT): results of a prospective, single-arm, open-label, phase 2 trial., PMID:40483169
Glucocorticoid treatment in early rheumatoid arthritis is independently associated with increased PCSK9 levels: data from a randomised controlled trial., PMID:40480650
Gender perspective in the management of psoriasis., PMID:40470625
Pharmacogenomics of TNF inhibitors., PMID:40469293
Predicting Clinical Response to Monoclonal TNF Inhibitors in Rheumatoid Arthritis: A Transcriptomic Approach Based on Transmembrane TNF Reverse Signaling and Nrf2 Activation., PMID:40428231
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
A systematic review of tumor necrosis factor-α blockers, anti-interleukins, and small molecule inhibitors for dissecting cellulitis of the scalp treatment., PMID:40383754
Influence of Rheumatoid Factors on the Efficacy of TNF Inhibitor Therapy in Patients with Rheumatoid Arthritis., PMID:40377857
Cardiovascular Outcomes of Disease-Modifying Antirheumatic Drugs in Rheumatoid Arthritis: A Review of the Current Evidence., PMID:40376321
Drug-associated infections and infestations in older adults with tumor necrosis factor-alpha inhibitors: a real-world retrospective and pharmacovigilance study., PMID:40371340
Real-World Insights From Türkiye: Biologic DMARDs Usage in Spondyloarthritis Patients With Chronic Kidney Disease., PMID:40358366
Aggregate Distributional Cost-Effectiveness Analysis of Biologics for the Treatment of Ankylosing Spondylitis in Chile., PMID:40329067
Novel Small-Molecule Treatment and Emerging Biological Therapy for Psoriasis., PMID:40299379
Racial Disparities in Utilization of Medications and Disease Outcomes in Inflammatory Bowel Disease Patients., PMID:40260307
Transitional and CD21- PD-1+ B cells are associated with remission in early rheumatoid arthritis., PMID:40259340
Recommendations for the use of DMARDs in pregnancy and reproductive health for patients with rheumatic disease: A scoping review., PMID:40256995
Triple biologic therapy for refractory Crohn's disease., PMID:40251965
A Case of Rectus Sheath Hematoma Caused by Self-Injection of Certolizumab Pegol., PMID:40196069
Discontinuation vs. continuation of concomitant methotrexate in patients with rheumatoid arthritis on certolizumab pegol: results from a randomised, controlled trial., PMID:40188084
Infection toxicity assessment of tumor necrosis factor α inhibitors in the treatment of IBD: a real-world study based on the US food and drug administration adverse events reporting system (FAERS)., PMID:40156444
Certolizumab-Induced Urticarial Vasculitis: A Case Report., PMID:40143392
Biological Disease-Modifying Antirheumatic Drugs Decrease Uric Acid Levels in the Sera of Patients with Psoriatic Arthritis., PMID:40136396
Pharmacovigilance study and genetic target prediction analysis of FDA adverse event reports (FAERS) for drug-induced sinusitis., PMID:40128146
Real-Life Analysis of Therapeutic Management and Its Correlation with the Dermatology Life Quality Index Score in 108 Patients with Pustular Psoriasis: An Italian Monocenter Study., PMID:40117644
Cost-effectiveness analysis of subcutaneous biosimilar tocilizumab in patients with rheumatoid arthritis in Spain., PMID:40113553
Rheumatoid Factor Predicts Long-Term Retention Associated With Effectiveness of Certolizumab Pegol in Patients With Rheumatoid Arthritis: A Two-Center Retrospective Study., PMID:40099319
Anti-Rheumatic potential of biological DMARDS and protagonistic role of bio-markers in early detection and management of rheumatoid arthritis., PMID:40091354
Certolizumab enhances spinal cord injury recovery in rats through inhibition of the TNF-α signaling pathway and neuronal apoptosis., PMID:40009347
Conducting a real-world study of Tumor Necrosis factor-alpha inhibitors-induced Systemic Lupus Erythematosus based on the FAERS database., PMID:40000785
Rheumatoid factors revisited in the age of biologic therapy., PMID:39982406
Risankizumab and Certolizumab Pegol Dual-Targeted Therapy for Crohn's Disease and Axial Spondyloarthritis: A Case Report., PMID:39981170
The use of aptamers as therapeutic inhibitors and biosensors of TNF-alpha., PMID:39971069
Tumor Necrosis Factor-Alpha Inhibitor Use and Malignancy Risk: A Systematic Review and Patient Level Meta-Analysis., PMID:39941759
Systematic review and bayesian network meta-analysis: comparative efficacy and safety of six commonly used biologic therapies for moderate-to-severe Crohn's disease., PMID:39911832
Certolizumab Pegol Treatment in Patients With Crohn's Disease: Final Safety Data From the SECURE Registry., PMID:39895830
Hidradenitis Suppurativa Treatment During Pregnancy and Lactation: Navigating Challenges., PMID:39887706
Management of Anal Fistula with Crohn's Disease., PMID:39882221
Exploring the neuroprotective potential of immunosuppressants in Parkinson's disease., PMID:39874798
Pharmacovigilance Pregnancy Data in a Population of Japanese Patients With Chronic Inflammatory Disease Exposed to Certolizumab Pegol., PMID:39812078
Certolizumab pegol in severe hidradenitis suppurativa in pregnancy: A case report., PMID:39803378
What is rheumatoid factor? From screening to personalized management., PMID:39792161
Real-World Persistence and Effectiveness of Upadacitinib versus Other Janus Kinase Inhibitors and Tumor Necrosis Factor Inhibitors in Australian Patients with Rheumatoid Arthritis., PMID:39757285
Effects of Ab501 (certolizumab mice equivalent) in arthritis induced bone loss., PMID:39754728
Impaired coagulation parameters in early RA are restored by effective antirheumatic therapy: a prospective pilot study., PMID:39740931
Effectiveness and Treatment Persistence of Vedolizumab Compared to Anti-Tumour Necrosis Factor-α in Patients With Crohn's Disease: A Systematic Literature Review and Meta-Analysis., PMID:39707930
Certolizumab pegol for plaque psoriasis in women of childbearing potential, pregnant or breastfeeding in clinical settings: One-year outcomes from the international noninterventional CIMREAL study., PMID:39699934
Pulmonary toxicity assessment of tumor necrosis factor α inhibitors in the treatment of IBD: a real world study based on US food and drug administration adverse events reporting system (FAERS)., PMID:39695351
Comparative Study of Adalimumab, Infliximab and Certolizumab Pegol in the Treatment of Cystoid Macular Edema Due to Behçet's Disease., PMID:39685848
Association Between Biologics and Janus Kinase Inhibitors With Inflammatory Bowel Disease as Paradoxical Reactions: A Real-World Assessment., PMID:39676727