Catalog No.
KAB94402
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Infliximab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Infliximab will be captured by immobilized Infliximab. After washing away any unbound substances, a biotin-labeled Infliximab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Infliximab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
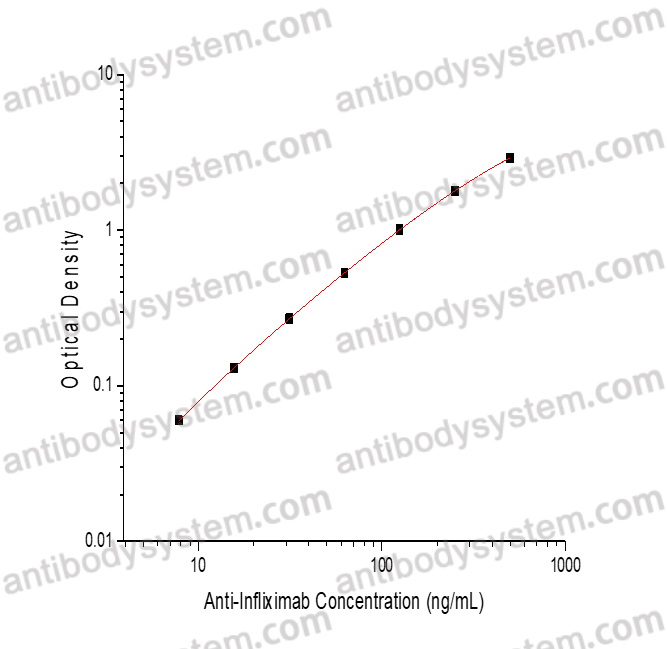
Range
7.81 - 500 ng/mL
Sensitivity
5.09 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
241.6
|
58.4
|
9.4
|
235.6
|
48.7
|
9.9
|
|
Standard deviation
|
8.1
|
1.8
|
0.9
|
14.2
|
1.8
|
0.6
|
|
CV (%)
|
3.4
|
3.0
|
9.9
|
6.0
|
3.7
|
6.4
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
TA-650,cA2,CAS: 170277-31-3
Native structure of the monoclonal therapeutic CD20 antibody ocrelizumab., PMID:40513927
Magnetic resonance spectroscopy biomarkers of anti-inflammatory interventions in mood disorders: A systematic review of clinical trials., PMID:40505984
Effectiveness and Safety of Advanced Dual-Targeted Therapy in Refractory Perianal Crohn's Disease., PMID:40504122
Post-traumatic ileal stenosis: a rare entity not to be confused with Crohn's disease., PMID:40503976
Curcumin-QingDai Combination as Treatment for Crohn's Disease: A Case Report., PMID:40503450
Serial 18F-Fluorodeoxyglucose Positron Emission Tomography to Assess the Efficacy of Infliximab in Steroid Refractory Cardiac Sarcoidosis., PMID:40499889
Comparative effectiveness of ustekinumab versus infliximab in the management of perianal fistulizing Crohn's disease: a retrospective study in China., PMID:40499579
Which first? Surgery or biologic therapy for ileocolic Crohn's disease in the real world., PMID:40498697
The Use of Intravenous Infliximab to Treat Cancer Related Fistulae: A Case Report in Home-Based Palliative Care Improving Quality of Life and Closure., PMID:40498467
Upadacitinib for refractory Behçet's disease with myelodysplastic syndrome and trisomy 8/9: a case report and mechanistic insights., PMID:40496858
Clinical characteristics and treatment of infantile Takayasu arteritis in the Chinese Han population: a single-center study., PMID:40495185
Development of thymoma without myasthenia gravis in a patient with radiographic axial spondyloarthritis treated with tumor necrosis factor-α inhibitors., PMID:40491307
Bibliometric analysis of global research trends on ultrasound in inflammatory bowel disease: A quickly developing field., PMID:40489862
Metabolism and Response to Stress Gene Signatures Reveal Ulcerative Colitis Heterogeneity and Identify Patients With Increased Response to Therapy., PMID:40488582
Infliximab Pharmacokinetics, Dosing, and Response in Hospitalized Patients with COVID-19 Pneumonia: A Secondary Analysis of a Multinational Randomized Clinical Trial (ACTIV-1 IM)., PMID:40488513
Efficacy and Safety of Vedolizumab with Glucocorticoids vs Infliximabin Severe Ulcerative Colitis: A Retrospective Study., PMID:40487981
Drug survival, long-term safety and efficacy of anti-TNF-alpha biosimilars: a monocentric retrospective study., PMID:40485573
Clinical spectrum of acute severe ulcerative colitis in the biologic era: a prospective cohort study from India., PMID:40485102
Pretransplant natural antibody levels identify a subset of deceased donor kidney transplant recipients that benefit from infliximab induction., PMID:40484132
Treatment sequences, outcomes, healthcare utilization, and costs in patients with inflammatory bowel diseases requiring advanced treatment-real world comparative effectiveness from German claims data., PMID:40481399
Tumor necrosis factor α inhibitor-induced alopecia in pediatric patients: a cohort of 20 patients and review of the literature., PMID:40481366
Anti-tumor Necrosis Factor-Alpha (TNF-α)-Induced Hypertrophic Lichen Planus: A Case Report., PMID:40470457
Pharmacogenomics of TNF inhibitors., PMID:40469293
Infliximab-Induced Immune Thrombocytopenic Purpura in an Ulcerative Colitis Patient., PMID:40469247
Comment on a Risk Stratification Tool for Relapse After Intravenous-to-Subcutaneous Switching of Infliximab in Patients With Inflammatory Bowel Diseases., PMID:40464796
Machine Learning Modeling for Predicting Infliximab Pharmacokinetics in Pediatric and Young Adult Patients With Crohn Disease: Leveraging Ensemble Modeling With Synthetic and Real-World Data., PMID:40459932
Switching from intravenous to subcutaneous infliximab in patients with immune mediated diseases in clinical remission., PMID:40454138
Higher induction and maintenance infliximab trough levels are associated with radiological perianal fistula healing in pediatric patients with Crohn's disease., PMID:40453222
Treatment of Autoimmune Enteropathy With Vedolizumab., PMID:40452653
Peripheral neuropathies associated with anti-tnf-α treatments: a systematic review and proposed recommendations., PMID:40447953
JAK-STAT inhibitors in noninfectious uveitis - A review., PMID:40434456
Optimizing Biologic Therapy for the Prevention of Post-Operative Recurrence in Crohn's Disease: Current Evidence and Future Perspectives., PMID:40427059
The Effects of the Biological Agents Infliximab, Vedolizumab, and Ustekinumab on Intestinal Anastomosis: An Experimental Study in Rats., PMID:40426907
Infliximab Biosimilar Utilization in a Large Pediatric Learning Health System., PMID:40426835
Obinutuzumab-Induced Inflammatory Bowel Disease-Like Colitis., PMID:40421460
Efficacy and safety of dual-targeted therapy for refractory inflammatory bowel disease: a retrospective case series from three tertiary general hospitals in China., PMID:40421289
Predictors of drug survival of biologics in hidradenitis suppurativa: A systematic review and meta-analysis., PMID:40419221
Comparative efficacy, safety and immunogenicity of biosimilars and their reference biologic drugs in ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials., PMID:40418343
The position of anti-Tumor Necrosis Factor agents for the treatment of adult patients with Crohn's disease., PMID:40418234
Association of the TNFRSF1B-rs1061622 variant with nonresponse to infliximab in ulcerative colitis., PMID:40414945
Exploring infliximab serum level variability in inflammatory bowel disease: Comprehensive analysis of patient subgroups and treatment outcomes "Infliximab trough levels variability in inflammatory bowel disease"., PMID:40414284
A 30-year experience in neuro-Behçet disease., PMID:40414042
Skin lesions and chest pain in a patient with Crohn's disease., PMID:40413034
Case report: Spontaneous splenic rupture concomitant with serum sickness induced by infliximab., PMID:40410001
Network Meta-Analysis: Efficacy of Biological Therapies and Small Molecules as Maintenance Therapy in Ulcerative Colitis., PMID:40407729
Ustekinumab in the Treatment of Crohn's Disease-A Narrative Review on Clinical Efficacy and Safety Profile., PMID:40407511
Effects of infliximab infusion on clinical symptom scores and serum cytokines in patients with inflammatory bowel disease., PMID:40400059
The Incidence and Management of TNF-α Inhibitor Induced Paradoxical Psoriasis in Children With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis., PMID:40400050
PErsistence and safety of subcutaneous infliximab 1 year after switch from intravenous route in IBD patients in REMission., PMID:40398832
Immunomodulatory Therapy Discontinuation for Reasons Other Than Efficacy in the Treatment of Ocular Inflammation., PMID:40397798