Catalog No.
KAD84001
Description
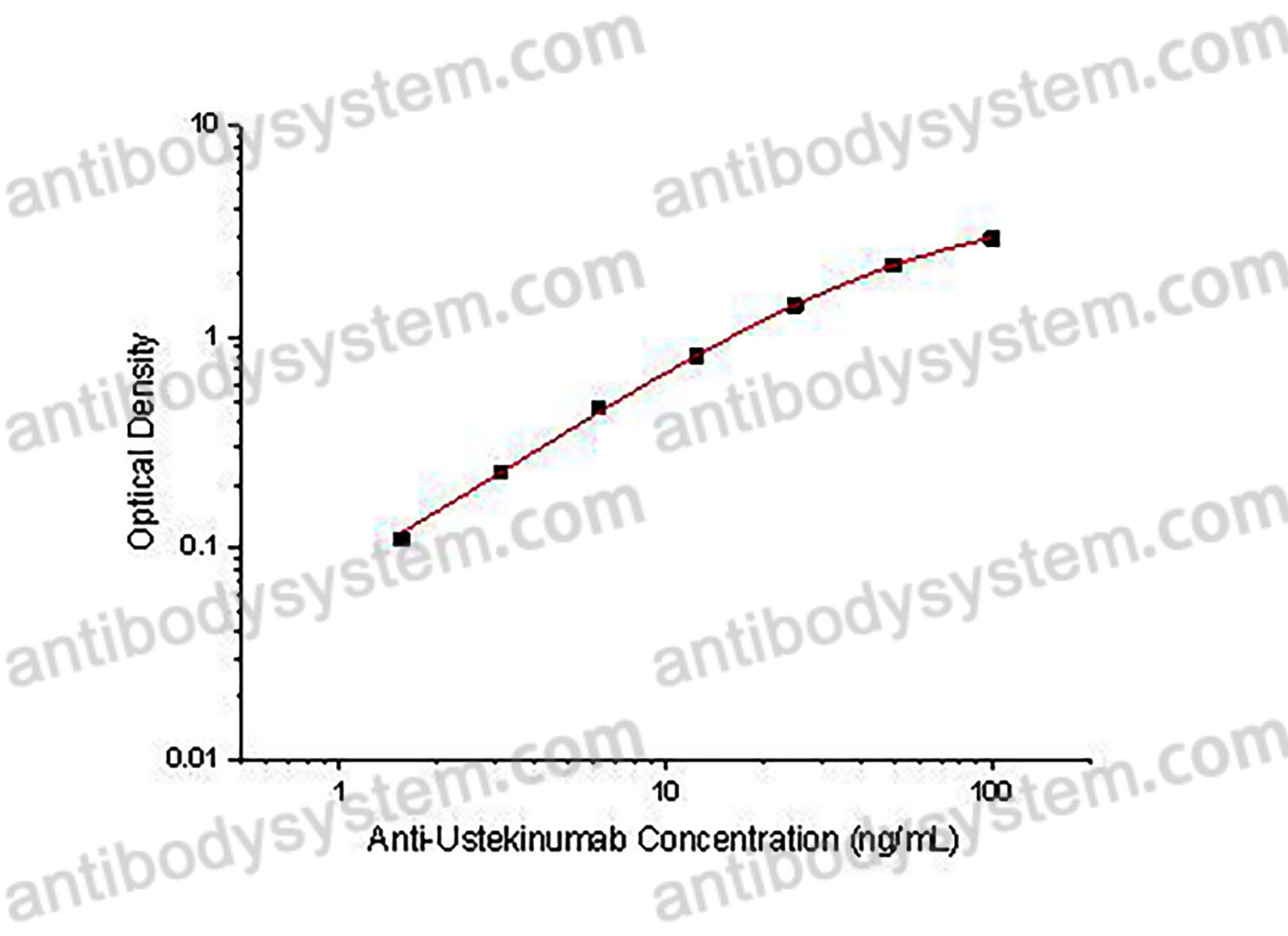
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Ustekinumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Ustekinumab will be captured by immobilized Ustekinumab. After washing away any unbound substances, a biotin-labeled Ustekinumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Ustekinumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Ustekinumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
1.56 - 100 ng/mL
Sensitivity
0.13 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
46.2
|
11.5
|
2.7
|
45.3
|
11.3
|
3.2
|
|
Standard deviation
|
2.0
|
0.2
|
0.1
|
1.5
|
0.6
|
0.4
|
|
CV (%)
|
4.4
|
1.6
|
2.6
|
3.3
|
5.0
|
12.8
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CNTO 1275, TT-20,CAS: 815610-63-0
Effectiveness and Safety of Advanced Dual-Targeted Therapy in Refractory Perianal Crohn's Disease., PMID:40504122
Comparative effectiveness of ustekinumab versus infliximab in the management of perianal fistulizing Crohn's disease: a retrospective study in China., PMID:40499579
The Effectiveness of second-and-third line biologics in perianal Crohn's disease - a multicenter propensity score-matched study., PMID:40490896
Wells syndrome: emerging triggers and treatments- an updated systematic review., PMID:40488888
Metabolism and Response to Stress Gene Signatures Reveal Ulcerative Colitis Heterogeneity and Identify Patients With Increased Response to Therapy., PMID:40488582
Impact of Obesity on the Long-Term Outcomes of Advanced Therapies in IBD: A Real-World Study in Taiwan., PMID:40487282
Impact of immediate drug optimization of ustekinumab on medium term targets in Crohn's disease: results from the multicentre retrospective real-life study MUST., PMID:40484747
Safety of guselkumab and ustekinumab treatment in patients with moderate-to-severe plaque psoriasis combined with latent tuberculosis or inactive hepatitis B virus infection: A retrospective multicenter observational study., PMID:40482822
Treatment sequences, outcomes, healthcare utilization, and costs in patients with inflammatory bowel diseases requiring advanced treatment-real world comparative effectiveness from German claims data., PMID:40481399
Interleukin-23 Inhibitors for Inflammatory Bowel Disease: Pivotal Trials and Practical Considerations., PMID:40465057
Guselkumab (Tremfya) - an IL-23 antagonist for Crohn's disease., PMID:40459404
Cancer Incidence in Patients with Ulcerative Colitis Naïve to or Treated with Thiopurine and Targeted Therapies- a cohort study 2007 to 2022 with comparison to the general population., PMID:40455688
Ustekinumab Dose Optimization in Ulcerative Colitis: Is More Always Better?, PMID:40447982
Cross-phenotype genome-wide association study supports shared genetic etiology between skin and gastrointestinal tract diseases., PMID:40441863
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Comparative Efficacy of Ustekinumab and Guselkumab in Improving Itch in Severe Psoriasis Patients., PMID:40432363
Advanced Therapies for Inflammatory Bowel Disease and Risk of Skin Cancer: What's New?, PMID:40427207
The Effects of the Biological Agents Infliximab, Vedolizumab, and Ustekinumab on Intestinal Anastomosis: An Experimental Study in Rats., PMID:40426907
Efficacy and safety of dual-targeted therapy for refractory inflammatory bowel disease: a retrospective case series from three tertiary general hospitals in China., PMID:40421289
Predictors of drug survival of biologics in hidradenitis suppurativa: A systematic review and meta-analysis., PMID:40419221
Short-Term Effectiveness of Ustekinumab in Crohn's Disease: Results from a Real-World Retrospective Multicenter Study in China., PMID:40417421
Use of ustekinumab as the treatment of choice in patient with corticodependent immune-mediated colitis secondary to pembrolizumab., PMID:40409592
Ustekinumab in the Treatment of Crohn's Disease-A Narrative Review on Clinical Efficacy and Safety Profile., PMID:40407511
Evaluating changes in baseline characteristics and drug utilisation pattern in patients with moderate-to-severe psoriasis: findings from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) Cohort., PMID:40402160
Complete Resolution of Pityriasis Rubra Pilaris With Targeted Treatment: A Case Report., PMID:40400847
The Incidence and Management of TNF-α Inhibitor Induced Paradoxical Psoriasis in Children With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis., PMID:40400050
The 'totality of evidence' and 'extrapolation' of SB17, a ustekinumab biosimilar., PMID:40396611
Systematic review and meta-analysis of the efficacy of biologic and targeted synthetic therapies in sarcoidosis., PMID:40393718
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
A systematic review of tumor necrosis factor-α blockers, anti-interleukins, and small molecule inhibitors for dissecting cellulitis of the scalp treatment., PMID:40383754
Induction with upadacitinib in Crohn's disease: real-world experience from an early-access program in Greece., PMID:40371210
Predicting ustekinumab treatment response in Crohn's disease using pre-treatment biopsy images., PMID:40366737
Real-World Insights From Türkiye: Biologic DMARDs Usage in Spondyloarthritis Patients With Chronic Kidney Disease., PMID:40358366
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease., PMID:40357993
Cost per remission for mirikizumab versus ustekinumab for moderately to severely active ulcerative colitis treatment from the United States commercial payer perspective., PMID:40351121
Innate and Adaptive Immunity is not Impacted by Inflammatory Bowel Disease Medications in Pregnant Women and Their Offspring., PMID:40349212
Alpha Fail: Ustekinumab to the Rescue After TNFα Failure in Patients with Moderate to Severe Crohn's Disease., PMID:40347351
Second-line strategies after anti-TNF failure in chronically active, moderate-to-severe ulcerative colitis: a retrospective, multicentre cohort study., PMID:40346848
Pyoderma Gangrenosum: A Retrospective Study Comparing TNF-α Inhibitors with Ustekinumab., PMID:40339789
Pharmacokinetic equivalence and comparative safety, tolerability, and immunogenicity of Biocon's ustekinumab (Bmab-1200) with EU-approved and US-licensed reference ustekinumab in healthy subjects: results from the Study to Test pharmacokinetic BioEquivalence of BiosimiLar ustekinumab to SteLARa (STELLAR-1)., PMID:40331766
Ustekinumab is effective in the treatment of linear psoriasis: a case report and literature review., PMID:40330485
The Effectiveness of Medical Therapies for Joint, Skin and Eye Extraintestinal Manifestations in IBD-An Umbrella Review., PMID:40329548
The real-world effectiveness of ustekinumab in patients with ulcerative colitis in the United States., PMID:40327500
One-Year Efficacy of Guselkumab Versus Advanced Therapies for the Treatment of Moderately to Severely Active Crohn's Disease: A Network Meta-Analysis., PMID:40327280
Mirikizumab (Omvoh) - an IL-23 antagonist for Crohn's disease., PMID:40324965
Prevalence of opportunistic infections in Syrian inflammatory bowel disease patients on biologic therapy: a multi-center retrospective cross-sectional study., PMID:40320559
Cutaneous and systemic improvements in psoriasis patients after different biologic treatments in a real-world longitudinal prospective study., PMID:40319105
Immunopathological and microbial signatures of inflammatory bowel disease in partial RAG deficiency., PMID:40314722